Behind the pages
Every year I organize and run the Pharmaceutical Cold Chain Management on Wheels course, which offers an exceptional 700 km tour of a comprehensive learning journey across the quality logistics. The course brings together both experts and novices from all over the world, who learn and share their experience during a ravishing 6-day travel across Turkey. Since its first inauguration in 2004, the programme of the course has been a living concept evolving around the most critical current issues and challenges of quality logistics, which are brought to a learning discussion and moderated by a team of top experts in the field of pharmaceuticals.
A comprehensive set of learning materials is prepared every year based on the anticipated demand.
During the 2015 course a participant suggested that the learning folder should include a glossary of key terms.
This was a good idea, and as the course director, my first reaction was sending a mail to all course participants asking for the list of terms they would like to see in such a glossary. In a week I received around 50 suggested terms. Naturally, they were all cold chain related with vaccine orientation. I thought that it would be useful to expand the glossary to cover the overall quality issue around pharmaceuticals and vaccines. As a result this volume includes the definitions of 730 terms.
Dictionaries define “quality” as “the standard of something when compared to other things like it” or as “how good or bad”. When we speak of “pharmaceutical/vaccine product quality”, there is much more that needs to be considered aside from the development, approval and manufacturing aspects. All products spend considerable periods of time at storage facilities, in transport between warehouses, at hospitals, pharmacies, health centres and at homes of end-users. Therefore just offering a “quality” product to the market is not enough. The product’s quality must be maintained throughout its life until it is consumed.
Cold chain is an area specific to time and temperature sensitive pharmaceutical products. Violating the rules of cold chain safety gravely affects the quality of a product. When we speak of “quality”, we should not only think about the product itself, but also everything else outside its box.
The spectrum of this glossary goes from product development and clinical trials to legislation around regulatory functions; from manufacturing to storage and dispatch activities and to post-marketing surveillance. It covers a comprehensive list of systems, procedures, and tools used at all levels that one way or another touch the issue of product quality.
What you have in your hand is not a simple glossary. Under many terms, in addition to their definitions, you will find additional information and perspectives on their uses. Wherever possible, tables, flow charts, decision tables, illustrations, sketches, and photographs are included.
Many colleagues asked me what process I followed in creating this volume.
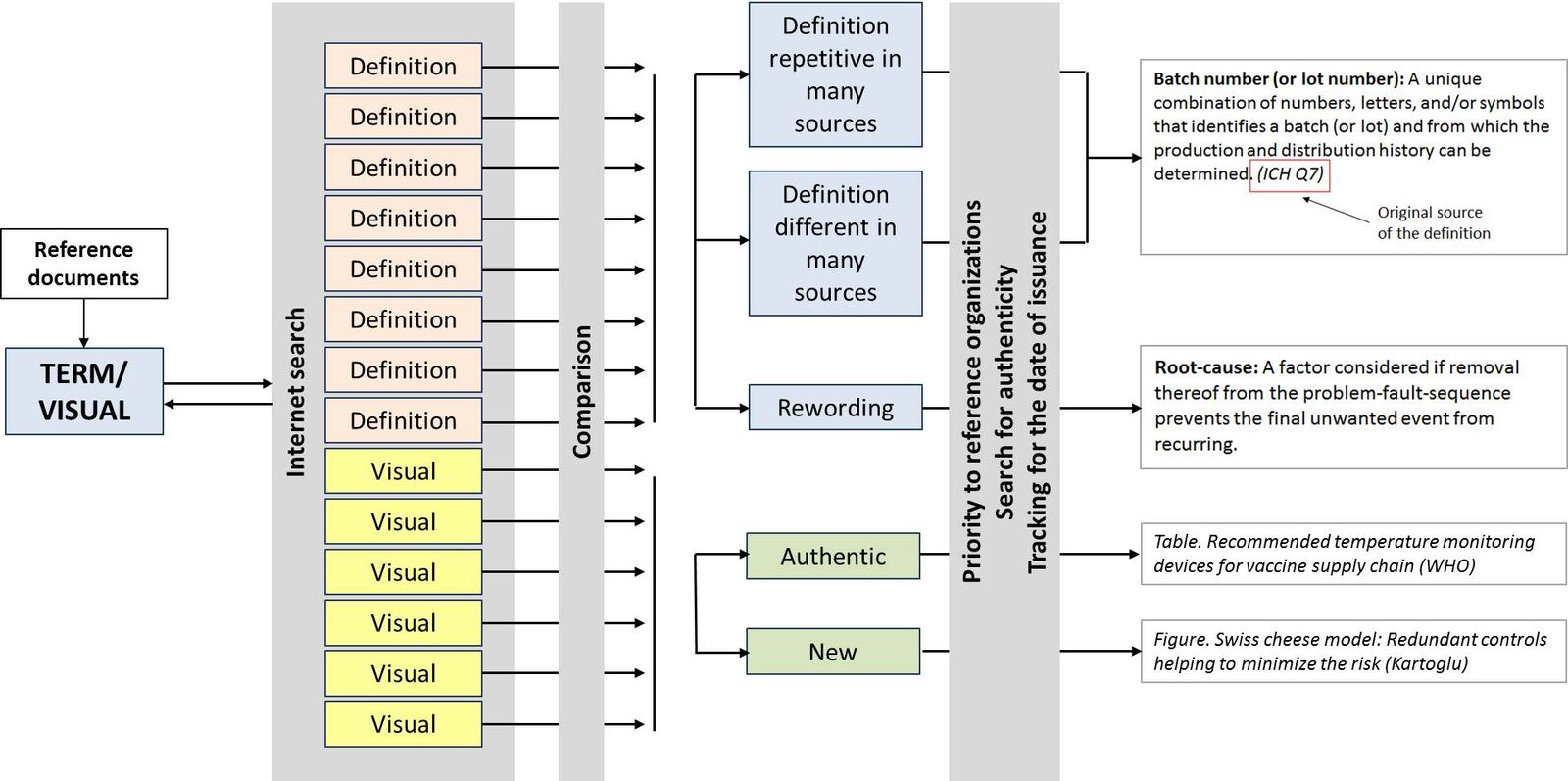
I started browsing the reference documents first. This helped me in expanding the pool of terms. For each term, I ran an extensive Internet search downloading all top 25 hits. These included pages from as common as Wikipedia to specific documents from reference organizations as well as books and peer-review articles. On the top of my list I put the definitions referenced by ICH, ISO, IATA, WHO, PDA and others.
The next step was to compare the definitions and find the overlapping ones, as well as deviations and in some cases gaps. Many definitions were repeated word by word in various sources without indicating the real source. In order to find out the origin, I worked on the publishing dates of the information involved. In some cases – but very seldom, based on everything I had in hand, I redefined the term. Some definitions included other terms within them. In the end I accumulated 730 terms.
I used a similar approach for the visuals. I printed out the existing visuals, and went through each and every single one to decide whether it was necessary to create a new one or to modify the existing one. In cases where the visual (be it table, flow-chart, graph, illustration or photograph) was authentic, I included it as it was. In other cases, I created the visuals myself or modified them from their original sources.
In the definitions, the italic references in parenthesis indicate the original source of the definition. My own or modified definitions do not have such indications. The same approach was also used for the visuals.
Here is how I would map out the process I followed.
This work is licensed under a Creative Commons (CC) Attribution-NonCommercial-ShareAlike 4.0 International License (CC BY-NC-SA 4.0). This license lets you reproduce, remix, tweak, and build upon this work non-commercially, as long as you credit the author and license your new creations under the identical terms. I believe in the Internet’s potential to drive a new era of development, growth, and productivity through universal access to research and education. The CC international license allows me to pursue this belief.
I worked on this document during my summer vacation of 2015, as well as during the long nights after work and the weekends between Switzerland and France. I dedicate this work to my loving wife Nellie and my daughter Deniz Nala, who are always there for me with their love and support. I feel grateful to my colleagues from WHO, UNICEF, the industry, national country programmes and national regulatory agencies with whom I discussed some of the terminology.
Ümit H. KARTOĞLU, MD, DPH
Collonge-Bellerive
October 2015

The users of this electronic publication are free to share (to copy, distribute, display and perform the work and make derivative works based on it only for noncommercial purposes); and to remix (to adapt the work) under the following conditions:
Attribution - The work must be attributed in the manner specified by the author or licensor (but not in a way that suggests that they endorse you or your use of work)