A
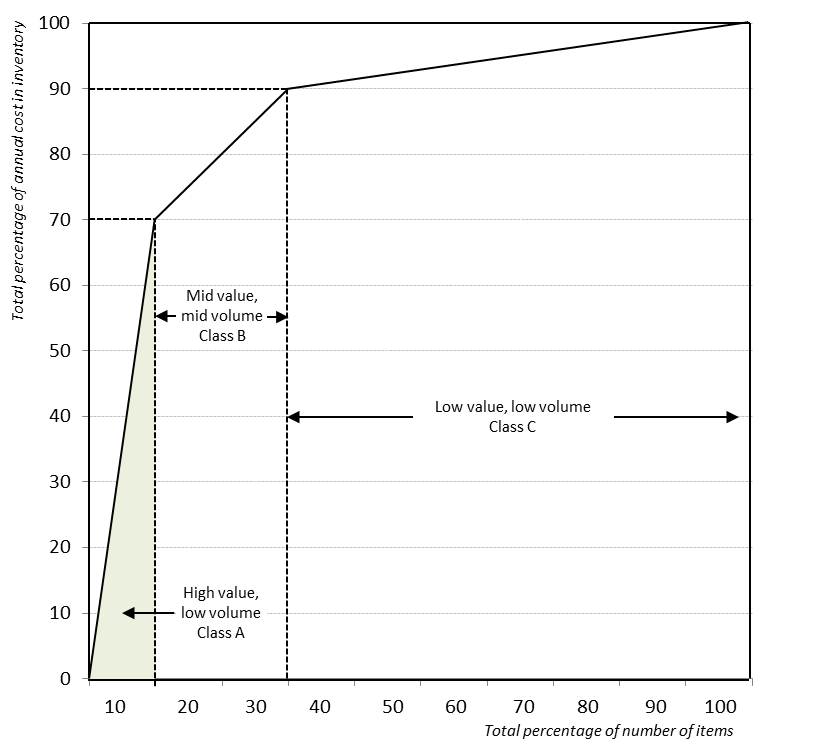
ABC analysis: Tool for reviewing stock movement, which categorizes items by the volume and value of consumption during a specific period of time, usually one year. Class A items – 10-20% of items, representing 75-80% of expenditures - are mostly high-volume, fast-moving medicines. Class B items are usually 10-20% of items, and 15–20% of expenditures. Class C items often represent 60-80% of the items but only about 5-10% of the total expenditures; these are the low-volume, slow-moving items. Thus, class C is a good place to look for items that might not be needed in stock at all times (WHO). See also VEN analysis.
Absorption cycle refrigerator: Refrigerator that uses a heat source such as gas or kerosene to drive the cooling system. A less efficient alternative to compression cycle refrigerators, absorption cycle refrigerators are most frequently used in areas with unreliable grid electricity. Currently there are no PQS prequalified absorption cycle refrigerators. (WHO)
Accelerated stability studies: Studies designed to determine the rate of change of vaccine properties over time as a consequence of the exposure to temperatures higher than those recommended for storage. These studies may provide useful support data for establishing the shelf-life or release specifications but should not be used to forecast real-time, real-condition stability of a vaccine. They could also provide preliminary information on the vaccine stability at early developmental stages and assist in assessing the stability profile of a vaccine after manufacturing changes. (WHO)
Accelerated testing: Studies designed to increase the rate of chemical degradation and physical change of an API or FPP by using exaggerated storage conditions as part of the stability testing programme. The data thus obtained, in addition to those derived from long-term stability studies, may be used to assess longer-term chemical effects under non-accelerated conditions and to evaluate the impact of short-term excursions outside the label storage conditions, as might occur during shipping. The results of accelerated testing studies are not always predictive of physical changes. (WHO)
Acceptable temperature range (for refrigerators): The acceptable temperature range for storing vaccine is +2°C to +8°C. However, WHO PQS prequalification programme indicates that transient excursions outside this range will be tolerated, within the following limits:- No excursion must exceed +20°C.
- No excursion must reach 0°C.
The cumulative effect of any excursions within the above range is assessed over the five day period of the day/night test. For this test, the calculated mean kinetic temperature (MKT) must remain within the range +2°C to +8°C when the default activation energy is set at 83,144 kJ per mol. using the recorded temperature data, an MKT figure is calculated for each sensor. The worst-case result determines the outcome of the test. Excursions in other tests are noted and must not exceed the defined upper and lower limits. (WHO)
Acceptance criteria: Numerical limits, ranges, or other suitable measures for acceptance of test results. (ICH Q6A)
Accident: An adverse outcome that was not caused by chance or fate. Most accidents and their contributing factors are predictable and the probability of their occurrence may be reduced through system improvements. (Canadian Patient Safety Dictionary)
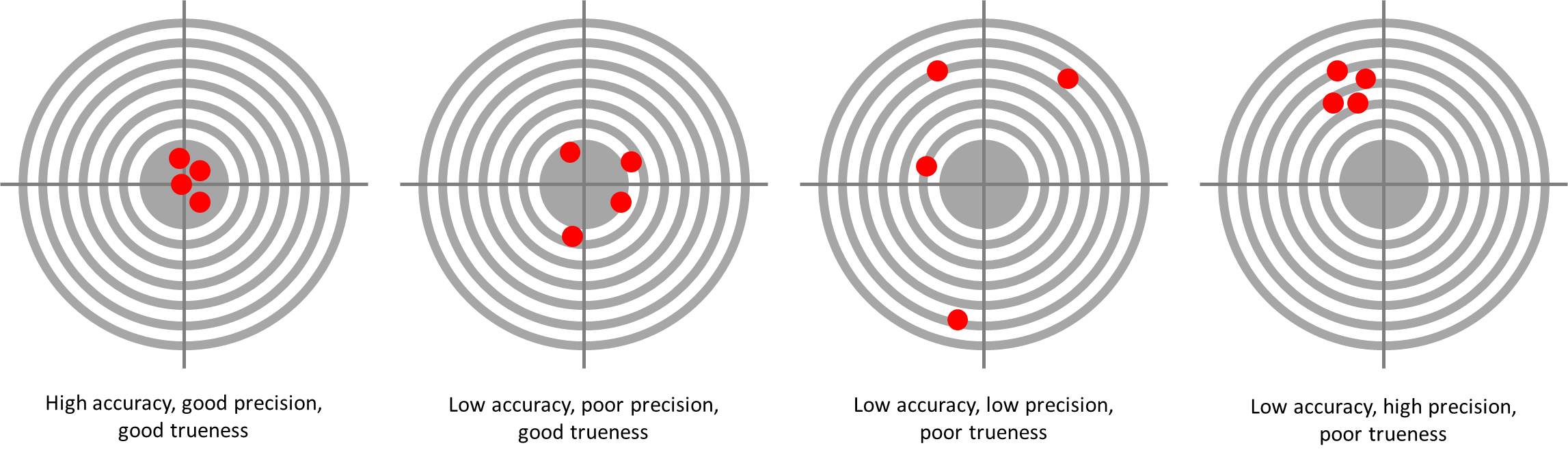
Accuracy: The closeness of a measurement to the true value. When the term is applied to sets of measurements of the same measurand, it involves a component of random error and a component of systematic error. In this case trueness is the closeness of the mean of a set of measurement results to the actual (true) value and precision is the closeness of agreement among a set of results. (ISO 5725-1)
The accuracy of an analytical procedure expresses the closeness of agreement between the value which is accepted either as a conventional true value or an accepted reference value and the value found. This is sometimes termed trueness. (ICH Q2/R1)
Accuracy is also used as a statistical measurement of how well a binary classification test correctly identifies or excludes a condition. In statistics it is also called as “rand accuracy” or “rand index”.
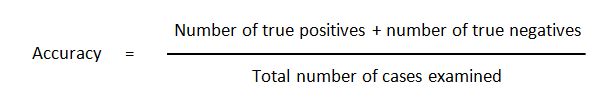
Active cooling: See active systems.
Active pharmaceutical ingredient (API): Any substance or mixture of substances intended to be used in the manufacture of a pharmaceutical dosage form and that, when so used, becomes an active ingredient of that pharmaceutical dosage form. Such substances are intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure and function of the body. (WHO)
Active systems: Externally powered or on-board powered systems using electricity or another fuel source to maintain a temperature-controlled environment inside an insulated enclosure under thermostatic regulation (e.g., cold rooms, refrigerators, temperature-controlled trucks, refrigerated ocean and air containers). (WHO)
Adjustments: Administrative corrections - e.g., a physical stock count that is different from quantity on stock keeping records. Also used for all losses within the facility (breakage, expiry, freezing, missing inventory, theft). (WHO)
Adjuvant: Substance that is intended to enhance relevant immune response and subsequent clinical efficacy of the vaccine, but does not in itself confers immunity. Adjuvants help activate the immune system, allowing the antigens - pathogen components that elicit an immune response - in vaccines to stimulate a response that leads to long-term protection. Most common adjuvants used in vaccines are aluminum hydroxide and aluminum phosphate. (WHO)
Administrative adjudication: A trial-like process in which the agency applies existing legal rules to particular situations. In addition to adopting regulations, administrative agencies also make law through the process of adjudication. Licensing decisions are a particular form of administrative adjudication, in which an agency grants authority to an individual or organization to engage in a particular activity. The drug approval process is an example of a licensing decision. In most countries, drugs may not be marketed or distributed until they have been approved by regulatory authorities for at least one indication. (WHO)
Advance shipping notice: A notification of pending deliveries, similar to a packing list, usually sent in an electronic format. It provides information to the destination’s receiving operations well in advance of delivery, and tends to impact logistics in reducing receiving cost, confirming accuracy and bringing flexibility. (WHO)
Advanced phase change materials (PCMs): Temperature stabilizing media (sometimes referred to as refrigerants), chemically engineered so that their latent heat of fusion occurs at a temperature other than 0°C, phasing from one state of matter to another (e.g., liquid to solid) at a pre-formulated temperature. Such materials typically comprise oils, salts, or paraffin. (WHO)
Adventitious agents: An infectious agent that is introduced into a therapeutic good during collection of raw materials or the manufacturing process. Adventitious agents include mycoplasma (a type of bacteria), viruses and agents that cause transmissible spongiform encephalopathies (prions). (TGA)
Adverse drug reaction (ADR): In the pre-approval clinical experience with a new medicinal product or its new usages, particularly as the therapeutic dose(s) may not be established: all noxious and unintended responses to a medicinal product related to any dose should be considered adverse drug reactions. The phrase responses to a medicinal product means that a causal relationship between a medicinal product and an adverse event is at least a reasonable possibility, i.e., the relationship cannot be ruled out. Regarding marketed medicinal products: a response to a drug which is noxious and unintended and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of diseases or for modification of physiological function.
The old term “side effect” has been used in various ways in the past, usually to describe negative (unfavourable) effects, but also positive (favourable) effects. It is recommended that this term no longer be used and particularly should not be regarded as synonymous with adverse event or adverse reaction. (ICH E2A)
Adverse event (AE): Any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment. An adverse event (AE) can therefore be any unfavourable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medicinal (investigational) product, whether or not related to the medicinal (investigational) product. (ICH E2A)
Adverse event(s) following immunization (AEFI): Any untoward medical occurrence which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine. The adverse event may be any unfavourable or unintended sign, abnormal laboratory finding, symptom or disease. If not rapidly and effectively dealt with, can undermine confidence in a vaccine and ultimately have dramatic consequences for immunization coverage and disease incidence. (WHO)
Also see AEFI investigation and causality assessment.
AEFI core variables: A set of critical variables recommended to properly managing AEFI information. This includes a unique identification of the report, the primary source of information, patient characteristics, details of the event(s) and vaccine(s) of interest and the possibility of collecting additional information if needed. (WHO)
AEFI core variables (WHO)
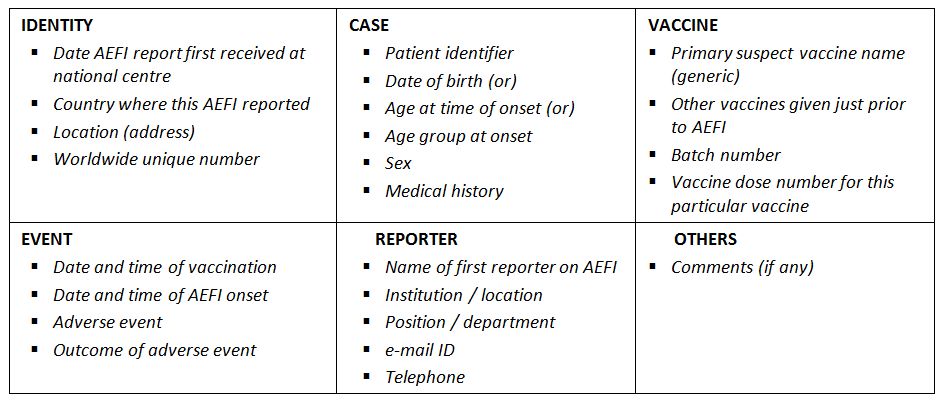
AEFI investigation: A systematic, standardized process to investigate reported serious adverse events following immunization to ascertain the underlying cause of the AEFI by:
- confirming a diagnosis and timing
- identifying details of vaccine(s) administered
- documenting the outcome of the reported adverse event
- determining whether the reported event is solitary or part of a cluster
- reviewing the operational aspects of the programme
Investigating AEFI clusters – suggested steps for identifying the most likely cause of a cluster of AEFI (modified from WHO)
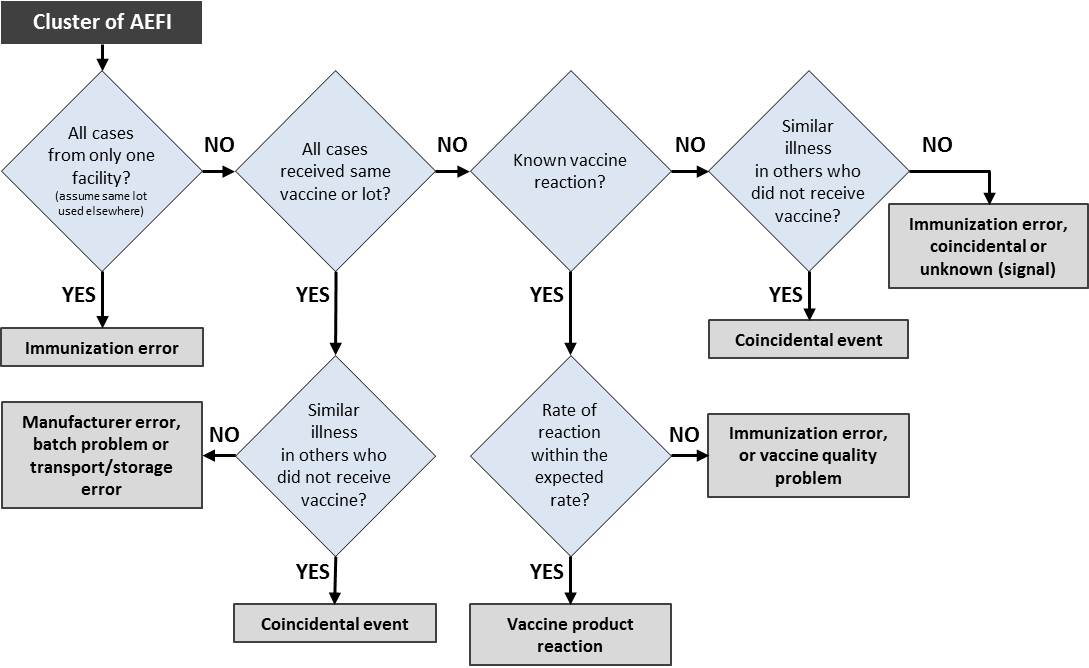
A detailed AEFI investigation to assess causality is necessary if:
- it is serious,
- it is part of a cluster,
- it is part of a suspected signal,
- it is a suspected immunization error,
- it appears on the list of events defined for AEFI investigation, or
- it causes significant parental or public concern.
Airliner: An inflatable insulating liner that converts a corrugated shipper into a cooler. It is manufactured from high-density polyethylene and low-density polyethylene inner and outer poly films with interior baffle films made from metalized poly film. An inflated airliner has a very low conductive rate and inhibits heat flow with this internal radiant barrier technology. Its mass consists mainly of the air used for inflation. Because it is shipped uninflated (flat), airliner occupies only 4% of the warehouse space required by traditional foam boxes, resulting in low shipping and storage costs.
Air waybill (AWB): A document made out by or on behalf of the shipper which evidences the contract between the shipper and the carrier(s) for carriage of goods over routes of the carrier(s). The AWB can be in the form of an airline air waybill with pre-printed issuing carrier identification, or neutral air waybill without pre-printed identification of the issuing carrier in any form. The industry is now transitioning from the use of the paper AWB to the electronic AWB. (IATA)
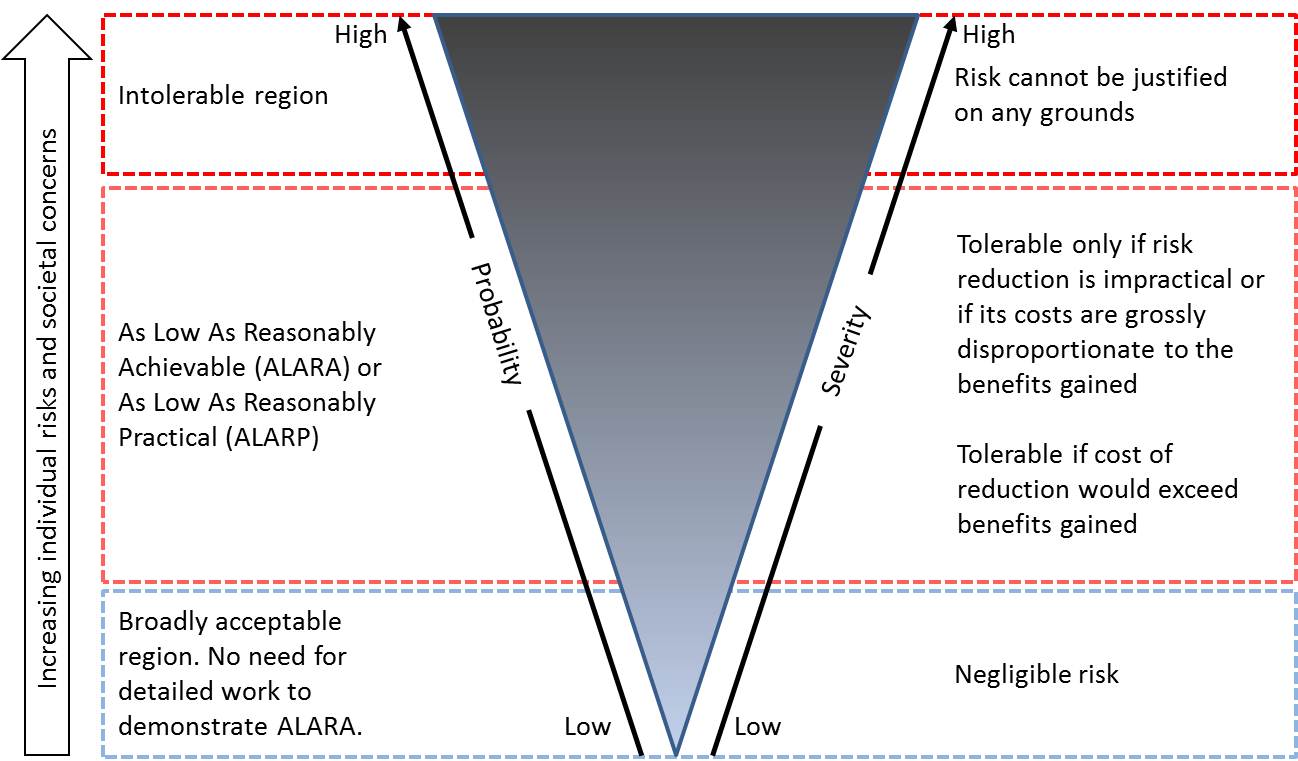
ALARA principle: ALARA stands for “As Low As Reasonably Achievable” and is a radiation safety principle for minimizing radiation doses and releases of radioactive materials by employing all reasonable methods. ALARA is not only a sound safety principle, but is a regulatory requirement for all radiation safety programmes. ALARA is also often applied in the pharmaceutical sector in the context of risk characterisation. In many cases ALARP (As Low As Reasonably Practical) principle is used over ALARA. The ALARP principle is that the residual risk shall be as low as reasonably practicable. To apply the principle it must be possible to show that the cost or practicality would be grossly disproportionate to the benefit gained. The principle arises from the fact that infinite resources (time, money, effort) could be used to reduce a risk but that is not achievable in the real world. It is not a simple quantitative measure of benefit against detriment. It is interlinked to the assessment if a risk is tolerable and/or controllable.
It is easier to identify unacceptable and acceptable risks. In identifying risks that fall in the ALARA/ALARP zone, the following factors should be taken into consideration as for the control measure defined:
- Effectiveness (Will it eliminate the risk or reduce the impact?)
- Benefits (What are the benefits of the controls?)
- Cost (How much will it cost?)
- Risks (Will the added control create new hazards?)
- Residual risks (What are the risks that remain after the controls being applied?)
ALARP principle: ALARP stands for “As Low As Reasonably Practical”. See ALARA.
Allocation system: See push system.
Alpha error: See type I error and validity.
Analytical procedure: The analytical procedure refers to the way of performing the analysis. It should describe in detail the steps necessary to perform each analytical test. This may include but is not limited to: the sample, the reference standard and the reagents preparations, use of the apparatus, generation of the calibration curve, and use of the formulae for the calculation. (ICH Q2/R1)
Ancillary packaging components: Packaging elements used to protect the TTSPP and support or enhance performance of the completed package. This may include retainers, dunnage, secondary protective packaging, and temperature data logging devices. (WHO)
Antibody : A protein substance produced by the immune system in response to a specific antigen that will identify and neutralize foreign material like bacteria and viruses, thus forming the basis of immunity. Each antibody recognizes a specific antigen unique to its target. Each antibody consists of four polypeptides - two heavy chains and two light chains joined to form a "Y" shaped molecule.
Antigen : Fragments or safe forms of pathogens that are used in vaccines, substances capable of stimulating an immunological response, such as the formation of antibodies. They may be proteins, polysaccharides, or complex lipids e.g., bacterial walls, the surface of erythrocytes, the protein capsule of viruses, the exotoxins and endotoxins of bacteria. The word “immunogens” reflects the fact that vaccines cause immune responses, not disease. Antigens responsible for initiating allergic reactions are called allergens.
Antigenic drift A mechanism for variation by viruses that involves the accumulation of mutations within the antibody-binding sites so that the resulting viruses cannot be inhibited well by antibodies against previous strains making it easier for them to spread throughout a partially immune population. Antigenic drift occurs in both influenza A and influenza B viruses. (WHO)
API starting material: A raw material, intermediate, or an API that is used in the production of an API and that is incorporated as a significant structural fragment into the structure of the API. An API Starting Material can be an article of commerce, a material purchased from one or more suppliers under contract or commercial agreement, or produced in-house. API starting materials are normally of defined chemical properties and structure. (ICH Q7)
Appreciation (so what) technique: An iterative question asking technique used to uncover factors that we might have ordinarily missed, also used to brainstorm solutions to problems as well as in root-cause analysis. Repetitive “so what?” question is asked to understand the implications of a fact until you draw all possible conclusions from it. Appreciation is similar to the 5 whys technique. The major difference is that it is often used to get the most information out of a simple fact or statement, while the 5 whys is specifically designed to reach down to the root of a problem.
Arrhenius equation: A formula for the temperature dependence of reaction rates. Chemical reactions are typically expected to proceed faster at higher temperatures and slower at lower temperatures. Quantitatively this relationship between the rate a reaction proceeds and its temperature is determined by the Arrhenius equation. At higher temperatures, the probability that two molecules will collide is higher. This higher collision rate results in a higher kinetic energy, which has an effect on the activation energy of the reaction. The activation energy is the amount of energy required to ensure that a reaction happens.
The effect of temperature on reaction rates using the Arrhenius equation can be calculated as follows:
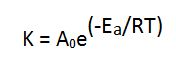
In this equation, A0 and Ea are experimentally determined constants specific to the reaction, and R is the universal gas constant (with a value of value of 8.314 x 10-3 kJ mol-1K-1). The activation energy, Ea, determines how the rate changes with temperature, T, which is expressed in degrees Kelvin.
For example, for a VVM30, the optical density changes essentially linearly with time and reaches its end-point by 30 days at 37°C, so the rate constant equals 1/30 per day at this temperature. The end-point is the stage where the difference between the optical density of the reference ring and the optical density of the active surface (center square) of the VVM reaches zero. The experimentally determined activation energy of 27.1 kcal/mole is used to determine the optical density change for any other time–temperature combination or to plot an Arrhenius chart of the end-point as a function of temperature.
Article 5 country: The main objective of the Multilateral Fund for the Implementation of the Montreal Protocol is to assist developing country parties to the Montreal Protocol whose annual per capita consumption and production of ozone-depleting substances (ODS) is less than 0.3 kg to comply with the control measures of the Protocol. Currently, 147 of the 196 parties to the Montreal Protocol meet these criteria (they are referred to as Article 5 countries). (WHO)
Article 58 (EMEA’s scientific opinion): A new European pharmaceutical legislation (Regulation 726/2004) excluded licensure of vaccines and other medicinal products for exclusive use outside the European Community. The new legislation generated considerable concern since licensure of priority vaccines for developing countries would become the responsibility of the NRAs of user countries, which, in the past, relied on the regulatory evaluation of the NRA of the country of origin to assure quality. (WHO)
To ensure that there was no disruption in the supply of vaccines and medicinal products that are important for developing countries and that there is no disincentive for the timely discovery and development of these products, a consultation and collaboration between EMEA and WHO led to the Article 58 in the new Regulation.
Article 58 establishes a mechanism whereby the EMEA may give a Scientific Opinion, in the context of cooperation with WHO, for the evaluation of certain medicinal products for human use intended exclusively for markets outside the Community. The procedure for implementation of Article 58 Scientific Opinion procedure, effective since May of 2005 begins with the request from the company to EMEA to assess the eligibility of the product for Scientific Opinion. WHO's input takes place in two instances:
- evaluation of eligibility of the vaccine for Scientific Opinion by EMEA;
- participation of experts proposed by WHO in the product evaluation process.
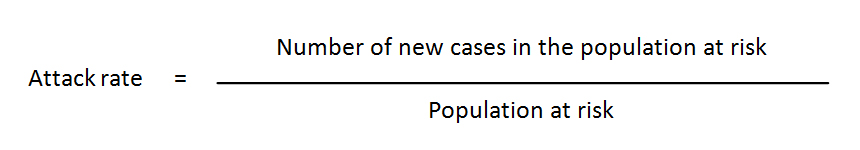
Attack rate: The biostatistical measure of frequency of morbidity, proportion of the population at risk exposed to an infectious agent who become (clinically) ill, used to project the number of victims to expect during an epidemic.
Audit: A systematic evidence gathering process. Audits must be independent and evidence must be evaluated objectively to determine how well audit criteria are being met. There are three types of audits:
- first-party (self-audits or internal audits)
- second-party (external audits carried out by customers)
- third-party (external audits carried by certification bodies)
In addition to this classification, ISO also distinguishes between combined and joint audits. When two or more management systems of different disciplines are audited together at the same time, it's called a combined audit; whilst two or more auditing organizations cooperate to audit a single organization it's called a joint audit.
Audit (of clinical trial): A systematic and independent examination of trial related activities and documents to determine whether the evaluated trial related activities were conducted, and the data were recorded, analyzed and accurately reported according to the protocol, sponsor’s standard operating procedures (SOPs), Good Clinical Practice (GCP), and the applicable regulatory requirement(s). (ICH E6/R1)
Audit (of transport): A systematic and independent examination of transport related activities and documents (e.g., freight bill) to verify its accuracy.
Audit certificate: A declaration of confirmation by the auditor that an audit has taken place. (ICH E6/R1)
Audit report: A written evaluation by the [sponsor’s] auditor of the results of the audit. (ICH E6/R1)
Audit trail: Documentation that allows reconstruction of the course of events. (ICH E6/R1)
Authorization holder: The person or company in whose name the marketing authorization has been granted. This party is responsible for all aspects of the product, including quality and compliance with the conditions of marketing authorization. The authorization holder must be subject to legislation in the country that issued the marketing authorization, which normally means being physically located in the country. (WHO)
Authorized person: See qualified person.
Autonomy (solar refrigerators): Maximum number of days during which the refrigerator can continue to maintain a full vaccine load at a temperature between +2°C and +8°C when the photovoltaic panels are not generating electricity. The autonomy of a solar refrigerator measures the ability of the equipment to store vaccine during periods of heavy cloud. (WHO)
Auxiliary equipment: Equipment mostly used in conjunction with the equipment to be qualified but not included in the qualification package. (WHO)
Average monthly consumption (AMC): Average consumption data of a product over a month. (WHO)
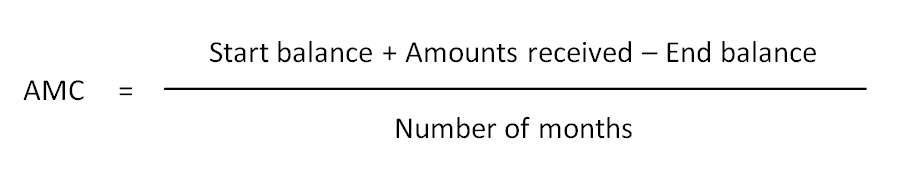
AMC can be derived either from inventory records or consumption data. “Issue data” substitutes consumption data in storage facilities. However, this should be used with some caution. In an allocation/push system “issue data” might be less accurate because dispatches are not based on actual consumption.

The users of this electronic publication are free to share (to copy, distribute, display and perform the work and make derivative works based on it only for noncommercial purposes); and to remix (to adapt the work) under the following conditions:
Attribution - The work must be attributed in the manner specified by the author or licensor (but not in a way that suggests that they endorse you or your use of work)