L
Label: All finished drug products should be identified by labelling, as required by the national legislation, bearing at least the following information (WHO):
- the name of the drug product;
- a list of the active ingredients (if applicable, with the International Nonproprietary Names (INNs)), showing the amount of each present, and a statement of the net contents, e.g., number of dosage units, mass or volume;
- the batch number assigned by the manufacturer;
- the expiry date in an uncoded form;
- any special storage conditions or handling precautions that may be necessary;
- the directions for use, and any warnings and precautions that may be necessary;
- the name and address of the manufacturer or the company or person responsible for placing the product on the market.
Written labels on the packaging permit the follow-up of a specific medicinal product by means of the batch number on the labels. It must be possible to follow the route of distribution of a product from the manufacturing process to its administration to the patient with the aim of locating and identifying products that are of potential risk (e.g. blood products, blood-derived products). They also mask the real identity of the medicinal product in clinical studies. This is extremely important in clinical trials in determining the real efficacy of a medicinal product in blinded studies. If the identity is masked by a code, it must be possible to disclose it at any time in a medical emergency. (WHO)
Labelling (for APIs and FPPs): The action involving the selection of the correct label, with the required information, followed by line-clearance and application of the label. A storage statement should be established for display on the label based on the stability evaluation of the API. Where applicable specific instructions should be provided, particularly for APIs that cannot tolerate freezing or excursions in temperature. Terms such as “ambient conditions” or “room temperature” should be avoided. (WHO)
Recommended labelling statements for active pharmaceutical ingredients and finished pharmaceutical products (WHO)
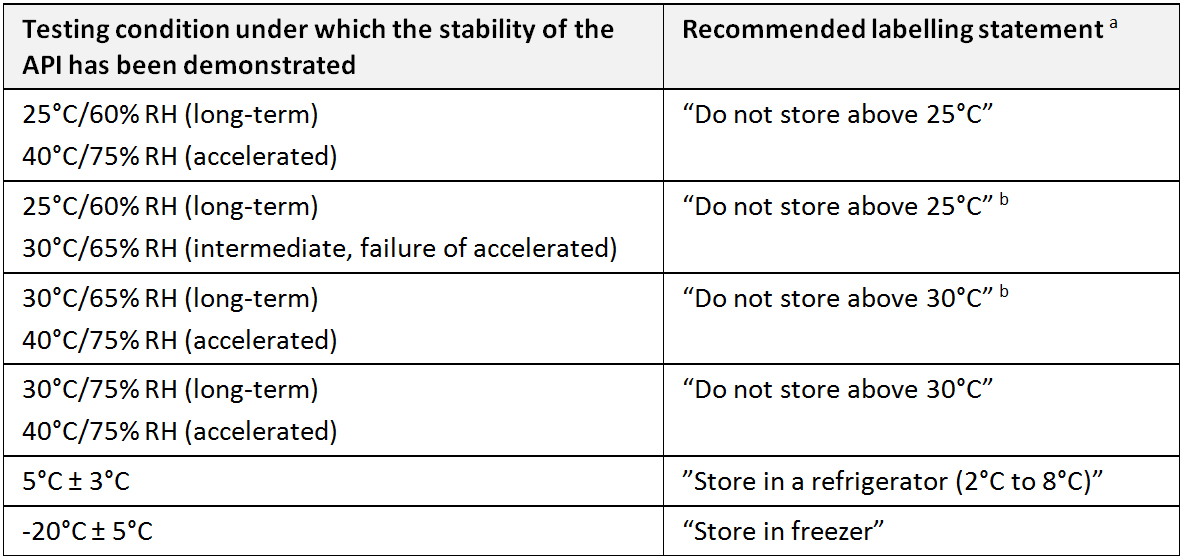
a During storage, shipment and distribution of the API, the current good trade and distribution practices (GTDP) for pharmaceutical starting materials are to be observed..
b “Protect from moisture” should be added as applicable.
In principle, FPPs should be packed in containers that ensure stability and protect the FPP from deterioration. A storage statement should not be used to compensate for inadequate or inferior packaging. Additional labelling statements that could be used in cases where the result of the stability testing demonstrates limiting factors are listed in the below Table.
Additional labelling statements for use where the result of the stability testing demonstrates limiting factors (WHO)
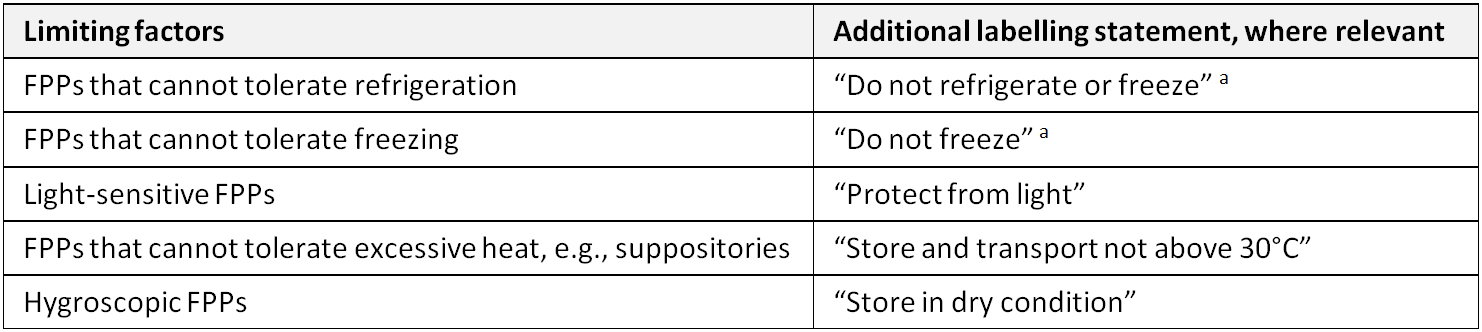
a Depending on the pharmaceutical form and the properties of the FPP, there may be a risk of deterioration due to physical changes if subjected to low temperatures, e.g., liquids and semi-solids. Low temperatures may also have an effect on the packaging in certain cases. An additional statement may be necessary to take account of this possibility.
Lanes: Transport routes from a point of origin to a destination.
Last mile: Last mile is the final leg of the supply chain that is between a service point and a customer, as it is often the least efficient link in the supply chain. By definition, it does not need to be a mile. For example, when you order a product from an online distributor located in another country and the product is sent directly to you, this is the last mile. In another example, when you ask your pharmacy for your prescription to be delivered to your home, this is the last mile. In health services, two approaches are used in last mile; active and passive. Immunization programme uses an active approach, when a child does not come for a scheduled vaccination session, the programme follows the person to reach and vaccinate. On the contrary, retail pharmacies use passive approach, they wait for patients to come with their prescriptions.
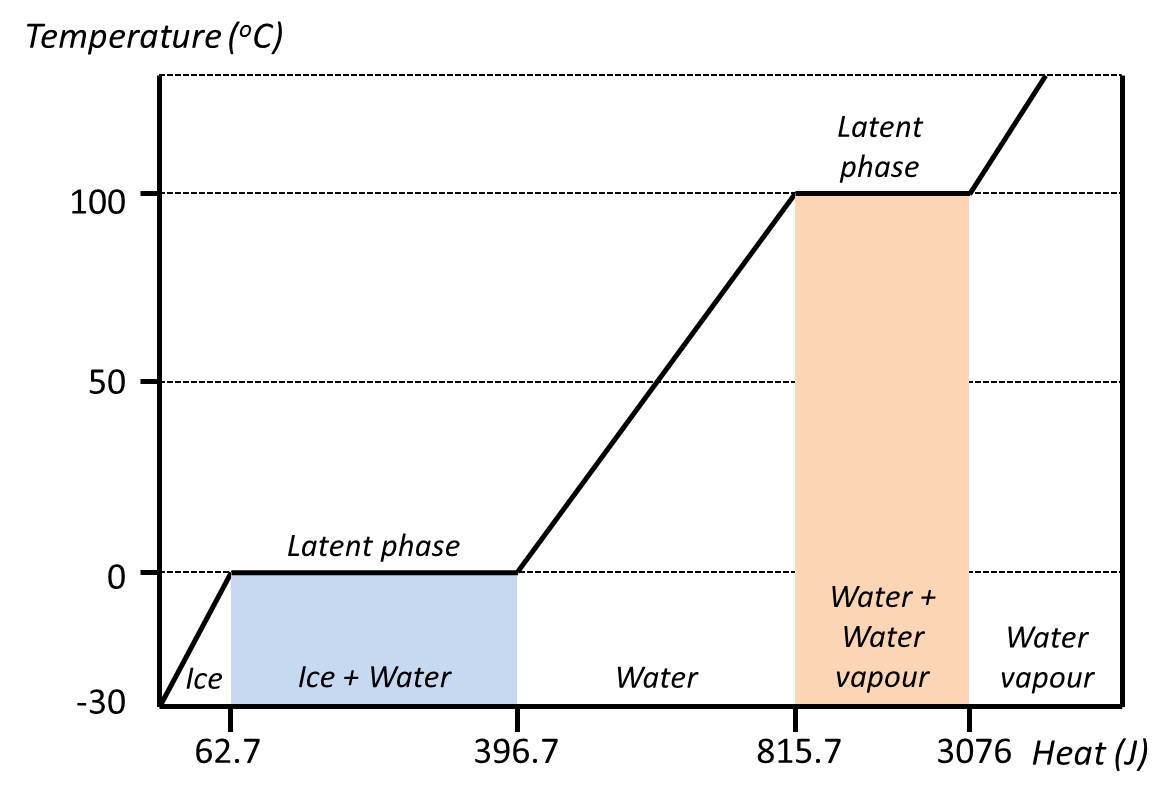
Latent heat: Characteristic amount of energy absorbed or released by a substance during a change in its physical state that occurs without changing its temperature. The latent heat associated with melting a solid or freezing a liquid is called the heat of fusion; that associated with vaporizing a liquid or a solid or condensing a vapour is called the heat of vaporization. (Encyclopædia Britannica)
While ice melts, it remains at 0°C, and the liquid water that is formed with the latent heat of fusion is also at 0°C. The latent heat is the major motive in using icepacks/PCMs for passive cooling.
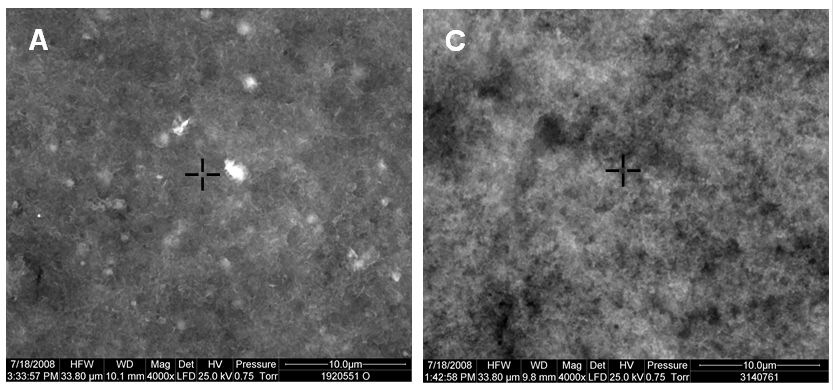
Lattice: Mesh like structure of bonds between aluminum adjuvant and the antigen in freeze-sensitive vaccines. The lattice is affected by freezing. Once frozen, the bond between the aluminum adjuvant and the antigen gets broken, and separated aluminum tends to form granules and conglomerates. See also shake test.
Scanning electron micrographs of lattice structure in non-frozen HepB (A) and DTP-HepB (C) vaccines(kept at +2°C to +8°C at all times)
Law: Any rule or standard of conduct that is recognized as binding and enforceable within a particular governmental system. Laws can exist in a variety of forms, including constitutions, statutes, administrative regulations, court decisions, executive orders, and other instruments. (WHO)
Lead time: The time between when new stock is ordered and when it is received and available for use. Lead time varies, depending on the system, speed of deliveries; availability and reliability of transport, and sometimes, weather. (WHO)
Lean manufacturing: A systematic method for the elimination of waste within a manufacturing system. Essentially, lean is centred on making obvious what adds value by reducing everything else.
Lean thinking: A term created by James P. Womack and Daniel T. Jones and is a business methodology aiming to provide a new way to think about how to organize human activities to deliver more benefits to society and value to individuals while eliminating waste. The basic insight of lean thinking is that if you train each staff to identify wasted time and effort in their own job and to better work together to improve processes by eliminating such waste, the resulting enterprise will deliver more value at less expense while developing every employee’s confidence, competence and ability to work with others.
Daniel T. Jones and James P. Womack

Legal manufacturer: The producer of the drug product or vaccine. A legal manufacturer is the natural or legal person with responsibility for the design, manufacture, packaging and labeling of a product or device before it is placed on the market under his own name, regardless of whether these operations are carried out by that person himself or on his behalf by a third party. (WHO)
Legally acceptable representative: An individual or juridical or other body authorized under applicable law to consent, on behalf of a prospective subject, to the subject’s participation in the clinical trial. (ICH E6/R1)
Lifecycle: All phases in the life of a product from the initial development through marketing until the product’s discontinuation.(ICH Q8)
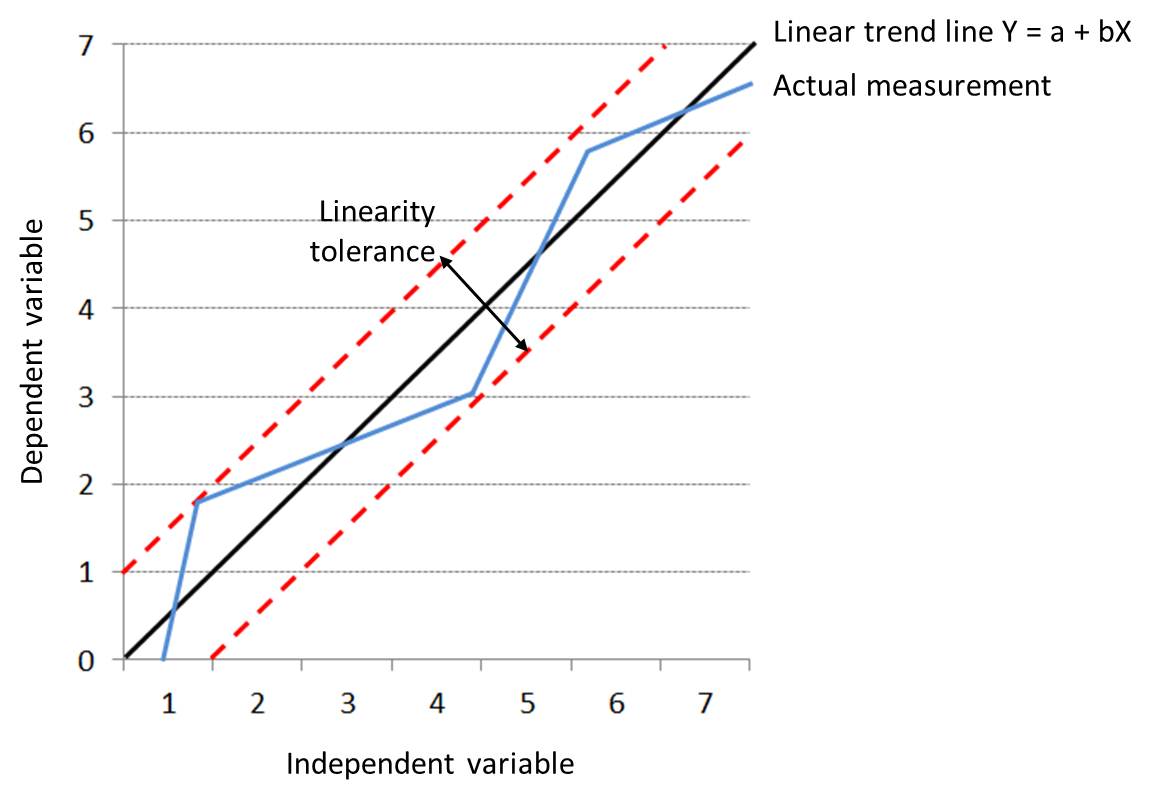
Linearity: A relationship of direct proportionality that, when plotted on a graph, traces a straight line (Y = a + bX). In linear relationships, any given change in an independent variable will always produce a corresponding change in the dependent variable.
Long-term stability studies: Experiments on the physical, chemical, biological, biopharmaceutical and microbiological characteristics of an API or FPP, during and beyond the expected shelf-life and storage periods of samples under the storage conditions expected in the intended market. The results are used to establish the re-test period or the shelf-life, to confirm the projected re-test period and shelf-life, and to recommend storage conditions. For APIs with a proposed re-test period or shelf-life of at least 12 months, the frequency of testing at the long-term storage condition should normally be every three months over the first year, every six months over the second year, and annually thereafter throughout the proposed re-test period or shelf-life. (WHO)
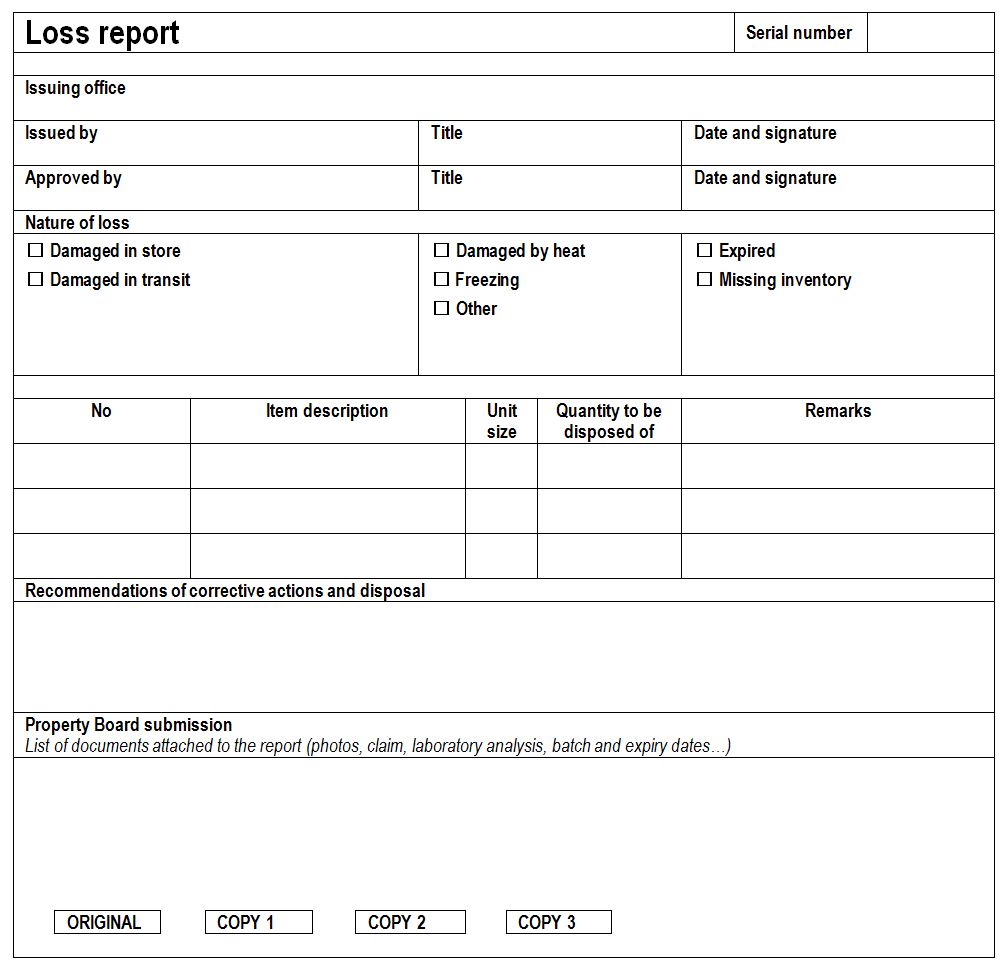
Loss report: Record of loss items issued for damaged, expired products as well as for missing inventory. Loss report is a critical report for accountability purposes. (WHO)
Losses: The quantity of stock removed from the pipeline for any reason other than consumption by clients (e.g., expiration and damage).
Lot: See batch
Lot number: See batch number.
Lot release: The process of evaluating each individual lot of a licensed product before giving approval for its release onto the market (WHO). This process is carried out for vaccines and other biologicals in most countries. General practices of release involves the review of manufacturer’s production data and quality control test results (product summary protocol) by the national regulatory authorities (NRA) and national control laboratory (NCL)s. This may or may not be supplemented by laboratory testing by the national control laboratory, or by an agency or contracted laboratory performing tests for the NRA. Lot release of vaccines by, as a minimum, review of a summary protocol and access to a laboratory are two of the essential functions of a NRA for assuring the quality of vaccines used in the immunization programme as defined by WHO.
Lot release certificate (LRC): A certificate issued by the manufacturing country’s national regulatory authority for every single batch of a product to be marketed, indicating that the batch of product has been examined and tested by the NRA/NCL and is in compliance with the approved specifications laid down in the relevant monographs of the pharmacopeia and in relevant marketing authorization (WHO). All shipments should be accompanied by the LRC issued by the regulatory authority of the producing country. In addition to the lot release certificates, producers usually include their own internal release documents with the shipping documents. At country level these documents are sometimes confused with the official lot release certificates issued by the NRA of the producing country. Manufacturers’ release documents and any other papers that may accompany a shipment do not replace and are not a substitute for the official lot release certificates issued by the NRA of the producing country.
Model certificate for the release of vaccines acquired by United Nations agencies (revised 1988)

Lot size stock: See working stock.

The users of this electronic publication are free to share (to copy, distribute, display and perform the work and make derivative works based on it only for noncommercial purposes); and to remix (to adapt the work) under the following conditions:
Attribution - The work must be attributed in the manner specified by the author or licensor (but not in a way that suggests that they endorse you or your use of work)