D
Damage indicator: Go/no-go type indicators that can sense dropping or tipping during shipment and temperature tools that tell you whether shipments were exposed to extreme temperatures. Shock and tilt indicators are mounted on a packaging container, the device visually alerts both handlers and the recipient. They may be single use in different sensitivities or multi-use resettable intended for large crates and shipments weighing over 500 pounds. The contents of a package will always experience less impact than the package because of dampening characteristics of the package. Applying the shock indicator directly on products allows you to more accurately determine if an impact was damaging to the product. They are available in different g-force ranges. Shock and tilt indicators provide indisputable evidence of mishandling, and visually alert recipient to inspect contents before acceptance. Shock and tilt indicators are usually used for fragile, sensitive, perishable or calibrated goods. See also impact indicator, shipping indicator, and g-force.
Dangerous goods: Solids, liquids, or gases that can harm people, other living organisms, property, or the environment. Dangerous goods include materials that are radioactive, flammable, explosive, corrosive, oxidizing, asphyxiating, biohazardous, toxic, pathogenic, or allergenic. Also included are physical conditions such as compressed gases and liquids or hot materials, including all goods containing such materials or chemicals, or may have other characteristics that render them hazardous in specific circumstances. Dangerous goods are often indicated by diamond-shaped signage on the material, container, or the building they are stored in. In many countries dangerous goods are known as hazardous materials (HAZMAT). Dangerous goods are divided into nine categories (and further down into some subcategories) on the basis of the specific chemical characteristics producing the risk:
- Explosives
- Gasses
- Flammable liquids
- Flammable solids
- Oxidizing agents and organic peroxides
- Toxic and infectious substances
- Radioactive substances
- Corrosive substances
- Miscellaneous
Also see Dangerous Goods Regulations and Hazard labels.
Dangerous goods regulations (DGR): A reference to the industry to help preparing and documenting dangerous shipments. DGR manual is the global reference for shipping dangerous goods by air and the only standard recognized by airlines. As for the global regulation of the dangerous goods, many specialized organizations (e.g., IATA, ICAO, IMO, OTIF) has issued regulations specific to their sector, based on the “UN Recommendations on the Transport of Dangerous Goods” issued by the United Nations Economic and Social Council , which form the basis for most regional, national, and international regulatory schemes.
Data and safety monitoring board (DSMB): An independent committee established by the sponsor to assess, at intervals, the ongoing scientific and ethical integrity of a study by reviewing and evaluating (unblinded) data and reports at regular intervals. The DSMB provides non-bonding recommendations to the sponsor regarding study modification, suspension, or termination. There is no fixed or harmonized international name for committees performing this function. Other names for committees performing the same or similar functions include, but are not limited to: Data Monitoring Committee (DMC), Independent Data Monitoring Committee (IDMC), Monitoring Committee (MC), Data & Ethics Monitoring Committee (DEMC), Safety Monitoring Committee, Study Monitoring Committee. (WHO)
Dead space (in syringes): Space occupied by the hub and the needle such that after the delivery of a full dose of a vaccine the liquid in these sections is wasted. Dead space can be minimized both by incorporating a plastic neck of a needle that fits within the neck of a standard syringe hub and by using an extended plunger that inserts into the neck of the syringe. By doing so, the liquid that is expelled from the syringe is maximum.
Declaration of Helsinki (DoH): A set of ethical principles regarding human experimentation developed for the medical community by the World Medical Association (WMA). Although DoH is not a legally binding document under the international law, it has been codified in or influenced both national and regional legislations and regulations throughout the world.
The declaration was originally adopted in June 1964 in Helsinki, Finland, and has since undergone seven revisions, most recently at the 64th WMA General Assembly in October 2013 in Fortaleza, Brazil. The current (2013) version is the only official one; all previous versions have been replaced and should not be used or cited except for historical purposes. For full text pdf version, please refer to http://www.wma.net/en/20activities/10ethics/10helsinki/DoH-Oct2013-JAMA.pdf
The declaration is morally binding on physicians, and that obligation overrides any national or local laws or regulations, if the Declaration provides for a higher standard of protection of humans than the latter.
Decrees: Legally-binding announcements issued by the head of government. In the United States, decrees are known as “executive orders” and are issued directly by the President. Executive orders are directed to federal agencies, not to the public in general. For example, Presidents George Bush and Barack Obama both issued executive orders establishing rules for the use of federal funds to support embryonic stem cell research. (WHO)
Deductive reasoning: Examining a general situation in order to understand specific, individual cases. In this regard, deductive reasoning moves from the general to the specific and involves “backward thinking” to find out what actions, or conditions contributed to the examined unwanted event. “What caused it to happen?” or “How did it happen?” is the major question in deductive reasoning. FTA risk assessment tool uses deductive reasoning.
Defects per million opportunities (DPMO): A measure of process performance used in six sigma. It is defined as:
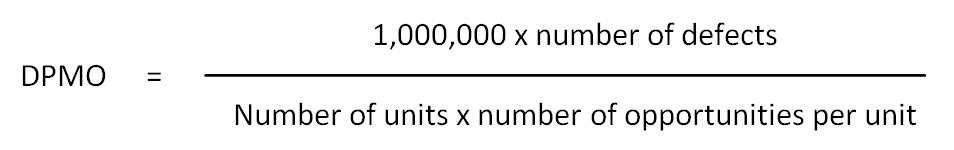
Denominator: The number below the line in a ratio; divisor; population at risk.
Dependent variable: The event studied and expected to change whenever the independent variable is altered. Dependent variable is measured by the experimenter. Dependent variable is also known as a "response variable", "regressand", "measured variable", "responding variable", "explained variable", "outcome variable", "experimental variable", and "output variable". In graphs, dependent variable is positioned in y-axis (vertical).
Design-build: A project delivery system used in the construction industry. The design and construction services are contracted by a single entity known as the design-builder or design-build contractor, typically for an agreed lump-sum price.
Design failure: The manner in which a system, subsystem, or part fails to meet its intended purpose or function.
Design qualification (DQ): The process of obtaining and documenting evidence that the premises, equipment and supporting systems and processes have been designed in accordance with the requirements for good manufacturing practices (GMP). (WHO)
Design space: The multidimensional combination and interaction of input variables (e.g., material attributes) and process parameters that have been demonstrated to provide assurance of quality. Working within the design space is not considered as a change. Movement out of the design space is considered to be a change and would normally initiate a regulatory post-approval change process. Design space is proposed by the applicant and is subject to regulatory assessment and approval. (ICH Q8)
Detectability: The ability to discover or determine the existence, presence, or fact of a hazard.(ICH Q9)
 Developing Countries Vaccine Manufacturers Network (DCVMN): Public health driven, international alliance of manufacturers, working to strengthen vaccine manufacturers through the provision of information and professional training programmes, technology improvements, innovative vaccine research and development, encouraging technology transfer initiatives, and educating the public about the availability of safe, effective and affordable vaccines for all people. DCVMN is established in the year 2000, sharing the vision of protecting all people against known and emerging infectious diseases, with the mission of increasing the quality and availability of vaccines affordable to all. Over the years the network grew reaching out today to include 44 vaccine manufacturers, in 16 countries and territories, producing and supplying over 40 different types of vaccines, in several presentations and using a variety of technology platforms totalling around 200 products. From those nearly 40 are prequalified by WHO. For details visit http://www.dcvmn.org/
Developing Countries Vaccine Manufacturers Network (DCVMN): Public health driven, international alliance of manufacturers, working to strengthen vaccine manufacturers through the provision of information and professional training programmes, technology improvements, innovative vaccine research and development, encouraging technology transfer initiatives, and educating the public about the availability of safe, effective and affordable vaccines for all people. DCVMN is established in the year 2000, sharing the vision of protecting all people against known and emerging infectious diseases, with the mission of increasing the quality and availability of vaccines affordable to all. Over the years the network grew reaching out today to include 44 vaccine manufacturers, in 16 countries and territories, producing and supplying over 40 different types of vaccines, in several presentations and using a variety of technology platforms totalling around 200 products. From those nearly 40 are prequalified by WHO. For details visit http://www.dcvmn.org/
Deviation: Departure from an approved instruction or established standard (USFDA). For installation qualification: any discrepancy between the installation specifications and the actual (as found) installation. For operational qualification: any discrepancy between the protocol and the actual performed test, test function methodology, testing equipment, and testing material.
DFSS: See DMADV.
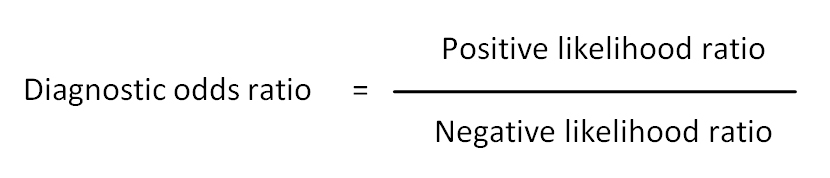
Diagnostic odds ratio: A measure of the effectiveness of a diagnostic test. It is defined as the ratio of the odds of the test being positive if the subject has a condition (disease) relative to the odds of the test being positive if the subject does not have the condition (disease). Also see validity.
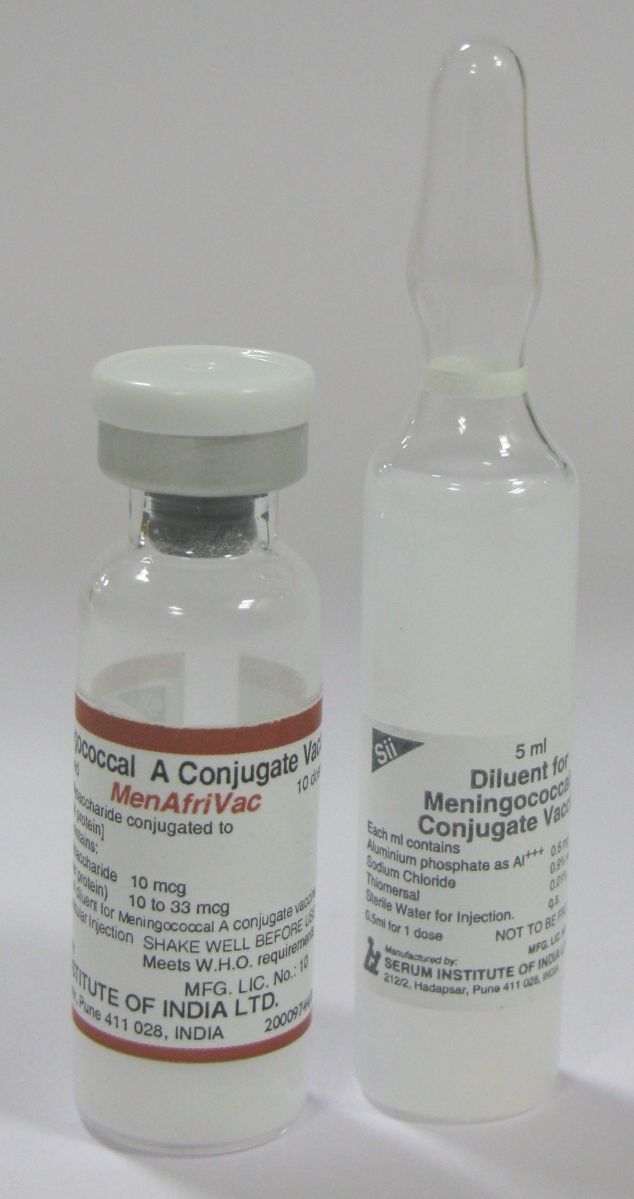
Diluent: A liquid used to reconstitute lyophilized (freeze-dried) vaccines for administration. Diluents are not just for dissolving vaccines, they are designed to meet an individual vaccine’s specific requirements in terms of volume, sterility, pH and chemical balance. Certain vaccine diluents include adjuvants and/or some antigens that are components of the final vaccine. For example, the diluent for Meningococcal A conjugate vaccine lyophilized (MenAfriVac) from Serum Institute of India Ltd., contains aluminium phosphate as adjuvant and thiomersal as preservative.
Diluents are not interchangeable. Each lyophilized vaccine must be reconstituted with its own diluent that is provided together with the vaccine. Using the wrong diluent, substituting normal saline, or using sterile water makes the vaccine ineffective and less able to provide protection against disease. Fatal AEFI cases have been reported due to incorrectly use of medications for reconstitution.
Direct access: Permission to examine, analyze, verify, and reproduce any records and reports that are important to evaluation of a clinical trial. Any party (e.g., domestic and foreign regulatory authorities, sponsor’s monitors and auditors) with direct access should take all reasonable precautions within the constraints of the applicable regulatory requirement(s) to maintain the confidentiality of subjects’ identities and sponsor’s proprietary information. (ICH E6/R1)
Dissipation: Result of an irreversible process taking place in inhomogeneous thermodynamic systems. Heat transfer is dissipative, because it is a transfer of internal energy from a high energy body to a low energy one.
Distributor: A receiving, warehousing and shipping site that delivers pharmaceutical products to pharmacies, hospitals, and healthcare centers. The distributor is an entity that buys noncompeting products or product lines, warehouses them, and resells them to retailers or direct to the end users or customers. There may be one or several distributors of pharmaceutical products between the manufacturer and the consumer. Sometimes the distributor is a commercial operation or company; at other times it could be a regional governmental or NGO facility. In any case, distributors receive relatively large shipments from manufacturers (or other distributors), warehouses them and sends smaller amounts of the product farther down the distribution chain. Distributors must ensure their facilities, procedures, and personnel do nothing to compromise the cold chain – and the quality of the product. (WHO)
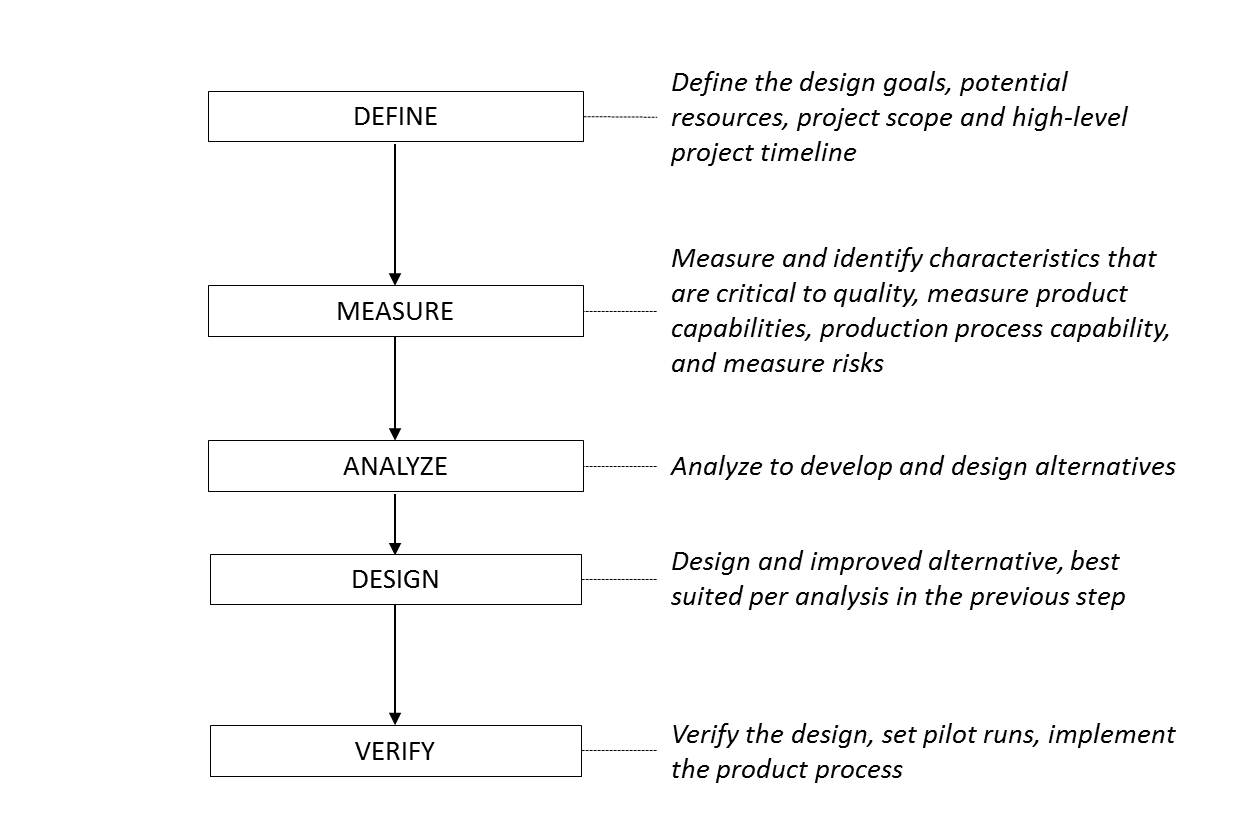
DMADV: (define, measure, analyze, design, and verify) - A project methodology, known as DFSS (design for six sigma), composed of five phases, mainly used for projects aiming at creating new product or process designs.
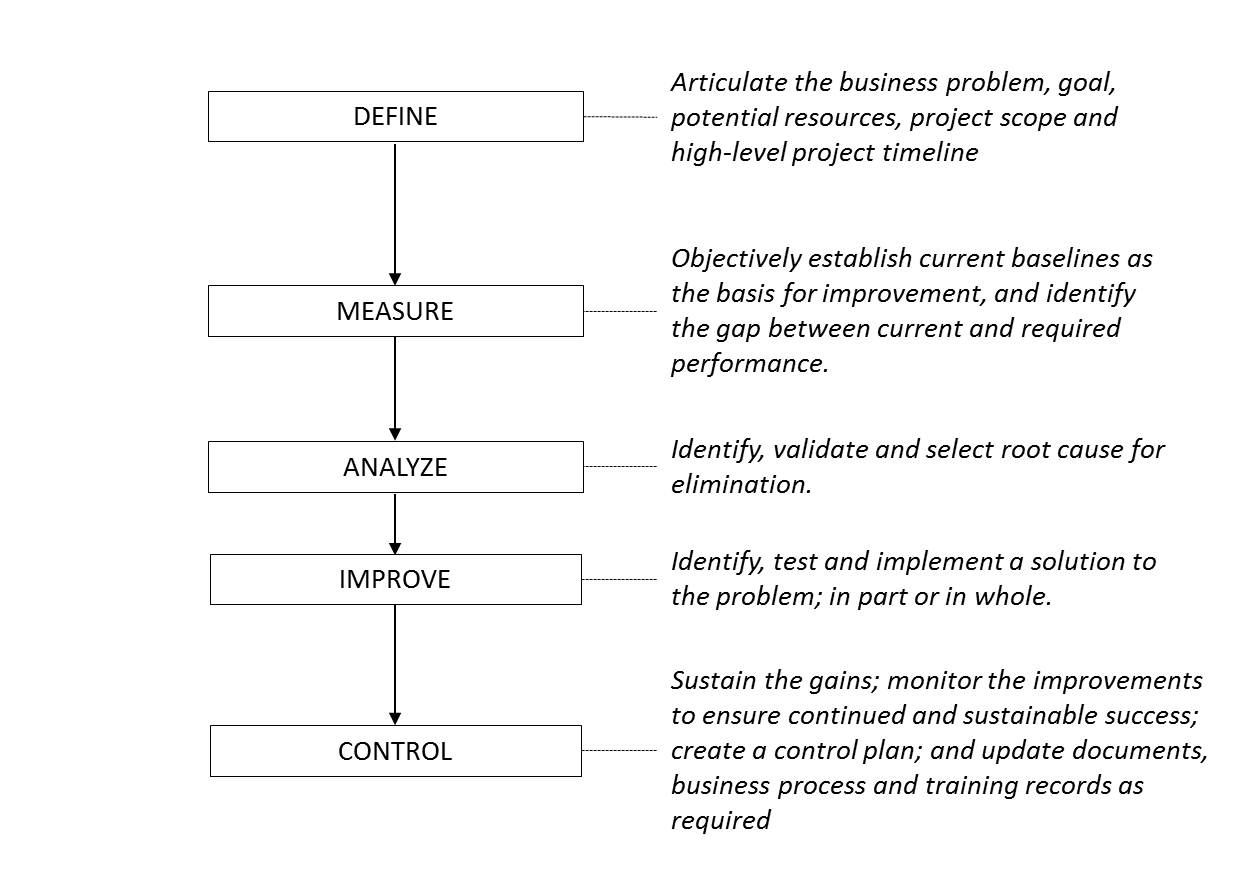
DMAIC: (define, measure, analyze, improve and control) - A core tool used to drive six sigma projects and data-driven improvement cycle used for improving, optimizing and stabilizing business processes and designs. DMAIC is used for projects aiming to improve an existing process.
Documentation: All records, in any form (including, but not limited to, written, electronic, magnetic, and optical records, and scans, x-rays, and electrocardiograms) that describe or record the methods, conduct, and/or results of a trial, the factors affecting a trial, and the actions taken. (ICH E6/Rev1)
Dosage form: The form of the FPP, e.g., tablet, capsule, elixir or suppository.
Various dosage forms

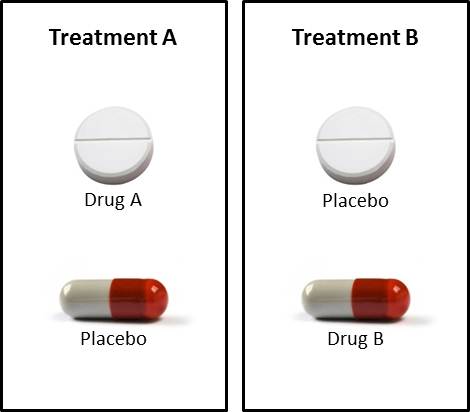
Double-dummy: A technique for retaining the blind when administering supplies in a clinical trial, when the two treatments cannot be made identical. Supplies are prepared for Treatment A (active and indistinguishable placebo) and for Treatment B (active and indistinguishable placebo). Subjects then take two sets of treatment; either A (active) and B (placebo), or A (placebo) and B (active). (ICH E9)
Drill-down technique: A process of breaking the problem down into its component parts.
Dropout (clinical trial): A subject in a clinical trial who for any reason fails to continue in the trial until the last visit required of him/her by the study protocol. (ICH E9)
Dropout rate: The percentage of children failing to complete a particular vaccine course in a given time period. It is calculated for vaccines that requires more than one dose. (WHO)
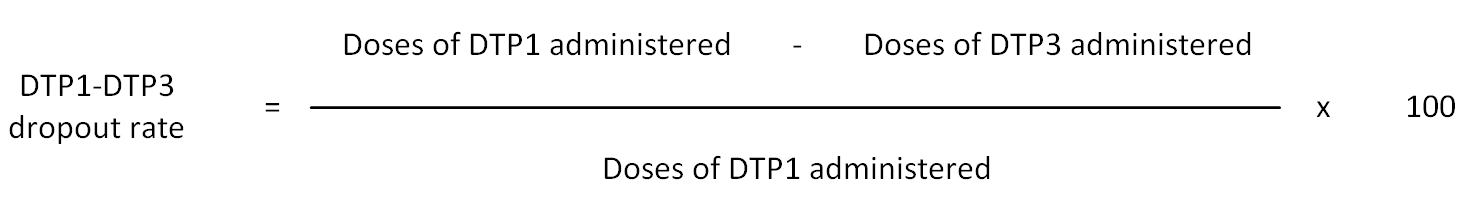
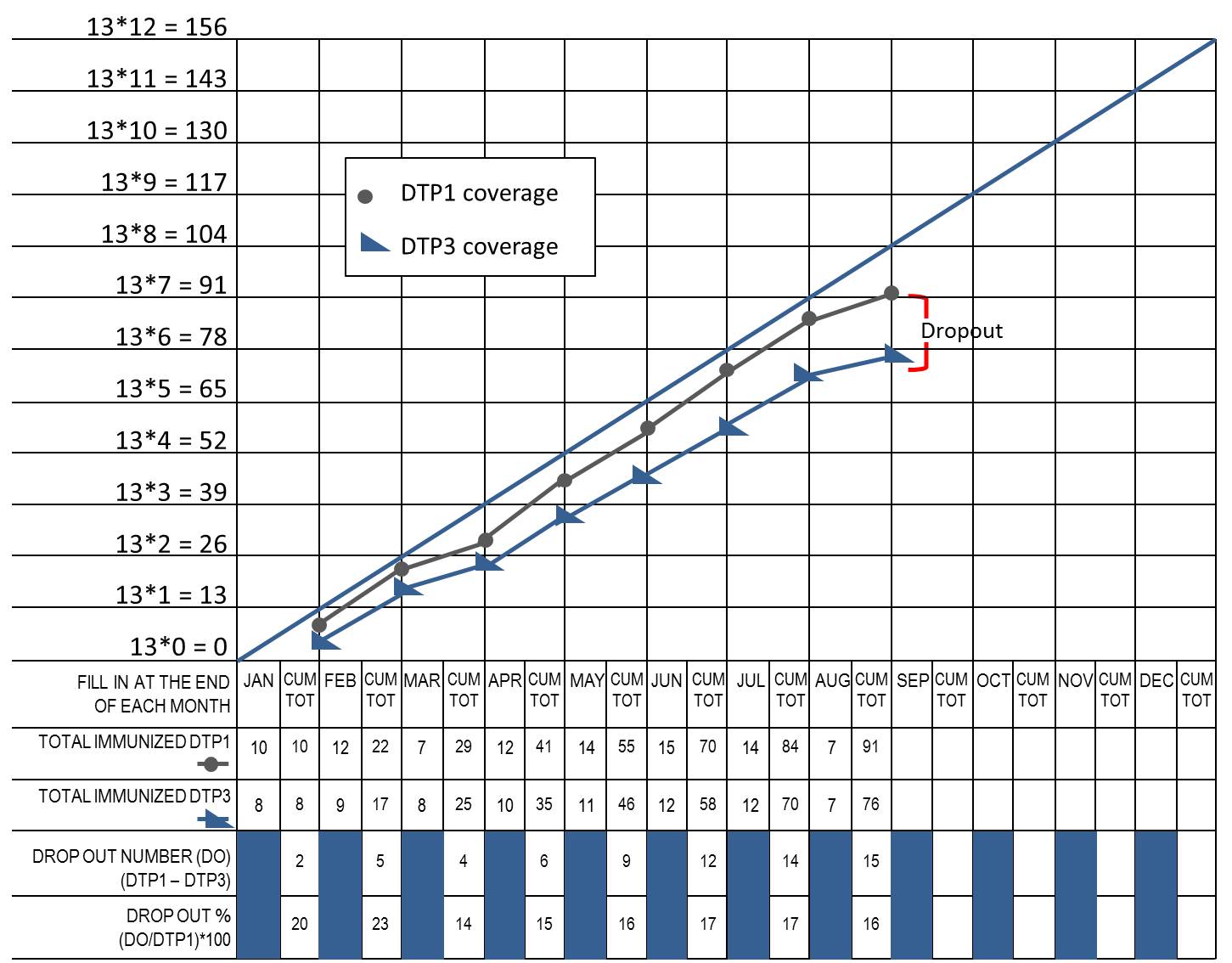
Dropout rates can be easily monitored visually in Immunization Monitor charts. However, it should be noted that in immunization monitoring charts “cumulative” figures are used. In this sense, as for the time period, for example, dropout at the end of March takes into account of cumulating of dropouts in January, February and March. The dropout rate can be easily visually monitored in these charts as the gap between the line of DTP1 and of DTP3 cumulative vaccinations.
Worked example of a monitoring chart for DTP1 and DTP3, WHO
Drug product: The drug product is the finished dosage form of the product. The drug product contains the drug substance(s) formulated with other ingredients in the finished dosage form ready for marketing. Other ingredients, active or inactive, may include adjuvants, preservatives, stabilizers, and/or excipients. For vaccine formulation, the drug substance(s) may be diluted, adsorbed, mixed with adjuvants or additives, and/or lyophilized to become the drug product (USFDA). See active pharmaceutical ingredient.
Drug substance: The drug substance is the unformulated active (immunogenic) substance which may be subsequently formulated with excipients to produce the drug product. The drug substance may be whole bacterial cells, viruses, or parasites (live or killed); crude or purified antigens isolated from killed or living cells; crude or purified antigens secreted from living cells; recombinant or synthetic carbohydrate, protein or peptide antigens; polynucleotides (as in plasmid DNA vaccines); or conjugates. For combination vaccines, each active substance, which will be pooled, combined with other antigens and formulated, should be described. (USFDA)Dunnage: Loose packing material used to protect TTSPPs from damage during transport.(WHO)

The users of this electronic publication are free to share (to copy, distribute, display and perform the work and make derivative works based on it only for noncommercial purposes); and to remix (to adapt the work) under the following conditions:
Attribution - The work must be attributed in the manner specified by the author or licensor (but not in a way that suggests that they endorse you or your use of work)