P
Package insert: A document provided along with a prescription or over-the-counter medication to provide additional information about the drug product. It is also known as “prescribing information” in the United States; and “patient information leaflet” for human medicines and “package leaflet” for veterinary medicines in Europe. National regulatory agencies determine the requirements for package inserts. A typical package insert includes the following information:
- Brand name and generic name of the product.
- Clinical pharmacology - explaining how the medicine works, how it is absorbed and eliminated, and what its effects are likely to be at different concentrations. Under this section, various clinical trials (studies) results may be given and/or explanations of the medication's effect on various populations (e.g. children, women, etc.).
- Indications and usage - uses (indications) for which the drug has been approved. Physicians legally can and often do prescribe medicines for purposes not listed in this section (so-called “off-label uses”).
- Contraindications - lists situations in which the medication should not be used.
- Warnings - covers possible serious side effects that may occur.
- Precautions - explains how to use the medication safely including physical impairments and drug interactions.
- Adverse reactions - lists all side effects observed in all studies of the drug.
- Drug abuse and dependence - provides information regarding whether prolonged use of the medication can cause physical dependence (if applicable)
- Overdosage - gives the results of an overdose and provides recommended action in such cases
- Dosage and administration - gives recommended dosage(s); may list more than one for different conditions or different patients (e.g., lower dosages for children)
- How supplied - explains in detail the physical characteristics of the medication including color, shape, markings, etc., and storage information.
WHO also issues requirements for its prequalified vaccine products. These package inserts come in several languages.
The very first patient package insert required by the USFDA was in 1968, mandating that isoproterenol inhalation medication must contain a short warning that excessive use could cause difficult breathing.
An example of a package insert in English (DTwP-HepB+Hib vaccine from LG Life Sciences Ltd. A WHO prequalified vaccine)

Packaging: Technology of enclosing or protecting products for distribution, storage, sale and use.
Packing slip: Transaction record sent with products that lists the names and quantities of each product shipped. Packing slip is usually paired with a receiving record. (WHO)
Packout: An assembled package that includes the product to be shipped (alternatively, simulated product in its primary packaging form used for its commercial presentation, the insulated shipper or container, any and all necessary auxiliary and/or associated components and ancillary packaging components such as temperature stabilizing medium, secondary packaging, partitions, bubble wrap, data loggers or other temperature monitoring units, and dunnage. (WHO)
Pallet: Wooden or plastic platform designed to be lifted by pallet jack or forklift truck. Typically used for storing and handling tertiary cartons.
Since pallets form an important part in the maritime industry, several norms and measures have been established by the ISO (International Organization for Standardization). Through such norms, it has been sought to bring the entirety of the freight operations which palletize their cargo consignments under a wider and common spectrum. The normative standardisation for pallets has been regulated in their sizing. Pallet sizes matter hugely while loading on palletized cargo ships as depending on the nature of the cargo, the optimal sized pallet is utilised to support the cargo consignments. See also ISO pallets and EUR pallet.
Wooden and plastic pallets (Andrea Crisante and nacykoa43, Shutterstock)


Pallet shipper: A combination of different products stacked together and shrink-wrapped on a pallet for shipment to a retailer. As for pharmaceuticals, it provides bulk holding of pharmaceutical products either in a single or multiple payload cavities with the advantage of increasing the payload thus reducing shipping cost.
RePak96™ 96hr insulated pallet shipper with two independent payload cavities (Cryopak)

Pandemic: An epidemic occurring over a very wide area, crossing international boundaries and usually affecting a large number of people. (WHO)
Passive cooling: See passive systems.
Passive systems: Systems which maintain a temperature-controlled environment inside an insulated enclosure, with or without thermostatic regulation, using a finite amount of preconditioned coolant in the form of chilled or frozen gel packs, phase change materials, dry ice or others. (WHO)
Performance qualification (PQ): The process of obtaining and documenting evidence that the premises, equipment and supporting systems, as connected together, will consistently perform in accordance with the approved process method and specifications. (WHO)
Performance, Quality and Safety (PQS): A WHO project to establish performance specifications, test procedures and prequalify a comprehensive range of immunization equipment, injection devices and other products needed for safe and effective immunization delivery.
The PQS on-line catalogue includes details of all immunization-related products currently pre-qualified by WHO for procurement by United Nations agencies. The catalogue replaces the old WHO/UNICEF Product Information Sheets (PIS), the last edition of which was published in 2000. Only products included in the PQS catalogue are now recommended to be purchased by UN agencies. The catalogue and the individual product data sheets are available on the internet only at:
http://apps.who.int/immunization_standards/vaccine_quality/pqs_catalogue/index.aspx
There is no hardcopy version. Each edition of the catalogue is date-stamped. It is updated regularly to ensure that the information it contains is current.
Product categorization for PQS prequalification scheme (WHO)
Following initial pre-qualification, all listed products must pass an annual review. This process takes account of feedback from purchasing agencies and users. Adverse reports may lead to product modifications, to suspension; or, in serious cases, to removal from the catalogue.
Perishable Cargo Regulations (PCR): A worldwide standard and an essential reference guide endorsed by IATA Live Animals and Perishables Board (LAPB) for all parties involved in the packaging and handling of temperature sensitive products from the health care and food sectors, including pharmaceutical products and non-hazardous biological materials. The PCR includes:
- Up-to-date airline and government requirements pertaining to the transport of perishable cargo
- Requirements on handling, marking & labelling
- Necessary packaging requirements
- Information on the necessary documentation required when transporting perishable cargo
- A comprehensive classification of 100’s of perishable commodities
Perishables: Liable to perish, especially naturally subject to quick deterioration or decay by time or exposition to adverse temperature and humidity (IATA). Perishable goods (such as fruits, flowers and vegetables) were among the first commodities carried by air.
Pharmaceutical equivalents: Products are pharmaceutical equivalents if they contain the same amount of the same active substance(s) in the same dosage form; if they meet the same or comparable standards; and if they are intended to be administered by the same route. Pharmaceutical equivalence does not necessarily imply therapeutic equivalence, as differences in the excipients and/or the manufacturing process can lead to differences in product performance. (WHO)
Pharmaceutical product: Any product intended for human use or veterinary product intended for administration to food producing animals, presented in its finished dosage form, that is subject to control by pharmaceutical legislation in either the exporting or the importing state and includes products for which a prescription is required, products which may be sold to patients without a prescription, biologicals and vaccines. Medical devices are not included. (WHO)
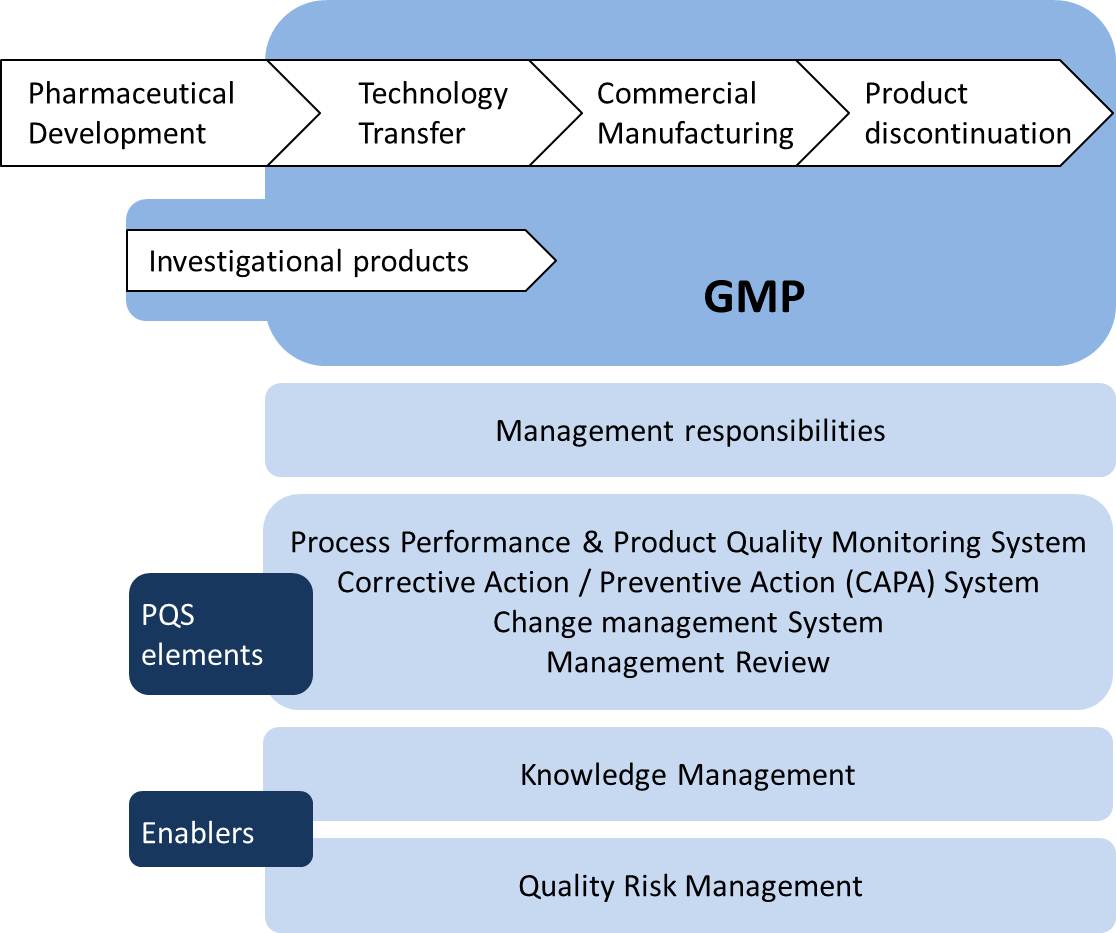
Pharmaceutical Quality System (PQS): Management system to direct and control a pharmaceutical company with regard to quality. (ICH Q10 based upon ISO 9000:2005 – revised by ISO 9000:2015)
Pharmacopoeia: A reference work for pharmaceutical drug specifications, a book containing directions for the identification of compound medicines, and published by the authority of a government or a medical or pharmaceutical society.
The first "Pharmacopoeia Helvetica" accomplished by the private initiative of the young Swiss Pharmacists Association (1865) and the latest one published by Swissmedic (2013)
The first "Pharmacopoeia Helvetica" accomplished by the private initiative of the young Swiss Pharmacists Association (1865) and the latest one published by Swissmedic (2013)


Pharmacopoeial text: The pharmacopoeial monographs, general test chapters, and analytical methods emanating from the three regional pharmacopoeias. (ICH Q4B)
Pharmacovigilance (vaccine): The science and activities relating to the detection, assessment, understanding, prevention and communication of adverse events following immunization, or of any other vaccine- or immunization-related issues (CIOMS).
The goal of vaccine pharmacovigilance is the early detection of and appropriate and timely response to adverse events following immunization in order to minimize negative effects to the health of individuals and lessen the potential negative impact on immunization of the population. Continuous risk-benefit assessment and risk management are integral to the vaccine pharmacovigilance process.
There is a very high level of safety required for vaccines. Elements to consider when conducting vaccine pharmacovigilance include the following (CIOMS):
- Vaccines are usually administered to healthy people, including infants.
- Vaccines may be administered to the vast majority of the population or of a birth cohort or to groups at high risk for disease complications.
- Subpopulations may be more susceptible to experience certain adverse events following immunization.
- The age at the time of immunization may coincide with the emergence of certain age-related diseases (e.g., neurodevelopmental disorders).
- Immunization with certain vaccines is mandated in some countries.
- The benefits of immunization may not be immediately visible, particularly if the target disease incidence is low.
- Due to the low acceptance of risks, intensive investigation of serious adverse events following immunization, even if rare, is necessary.
- Non-serious adverse events following immunization also should be carefully monitored because they may signal a potentially larger problem with the vaccine or immunization, or have an impact on the acceptability of immunization in general.
- Appropriate methods are needed to detect and assess any potential causal association of serious, rare, and/or delayed adverse events, or of adverse events in subgroups, with immunization.
- Consideration of dechallenge and rechallenge differs for vaccines compared with other medicinal products. Vaccines are frequently administered only once or with long intervals, and serious adverse events following immunization often prevent further vaccine administration. Dechallenge may not be applicable to vaccines, given their long-term immunological effects, and rechallenge information is only rarely available.
- Vaccines are often administered concomitantly with other vaccines, making causal attribution to a specific vaccine difficult.
- The administration of live vaccines can lead to disease caused by the attenuated organisms in vaccinees or their contacts; this should be differentiated from coinciding natural infection.
- Vaccines are complex biological products, which may include multiple antigens, live organisms, adjuvants, and preservatives. Each component may have unique safety implications. Variability and (even small) changes in the manufacturing process may have impact on quality, protective effect, and safety. Batch information is of crucial importance.
- New vaccines are increasingly based on new production and administration technologies, with new adjuvants and alternative routes of administration, necessitating adapted safety monitoring systems.
- Depending on the mode and extent of use of a vaccine, it may elicit a degree of herd immunity to a specific disease. When assessing the risk-benefit of a vaccine, herd immunity effects as well as individual protection need to be taken into account.
- Effective communication regarding the safety of vaccines and immunization is challenging. Despite strong evidence that a serious adverse event is not related to immunization, perceptions of harm may persist and may potentially have a negative impact on immunization of the population.
Physical inventory: A count of all the commodities on stock to verify that the amount that is actually stored is the same as the quantity listed in the stock-keeping records. Operations are usually shut-down during a physical inventory. Sometimes errors occur in counting the quantities of commodities entering or leaving a store. A regular physical check is the only way to ensure that stock records and running balances are accurate, matching and complete. If the result of counting a stock item is different from that shown in the record, the stock should be counted again to ensure there was no counting error. If a second count gives the same result as the first, the stock record is probably in error, and must be corrected. (WHO)
Sample physical inventory report for a vaccine storage facility (WHO)
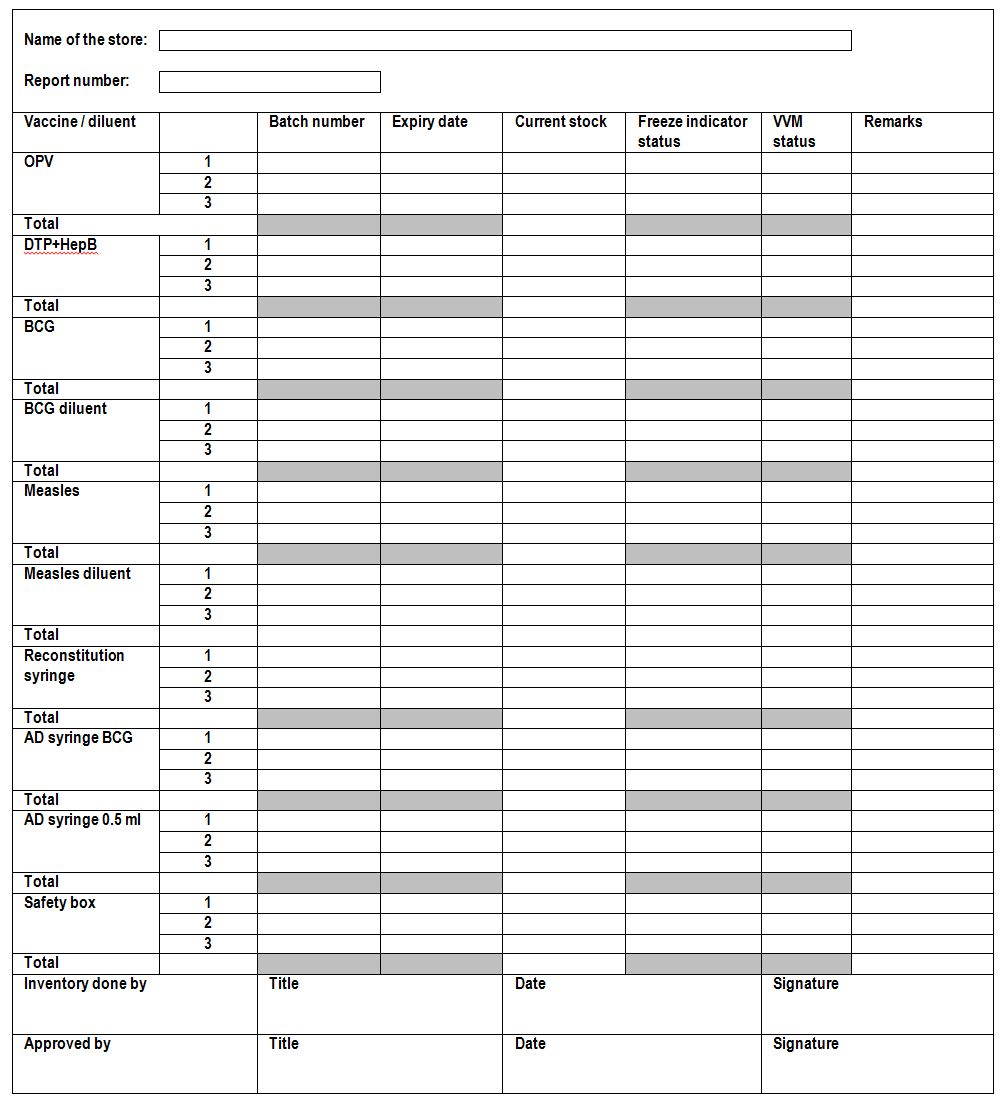
- If more items are counted than are recorded: Record the additional amount in the loss/adjustment column of batch and inventory control cards with an explanation of the reason in the remarks column.
- If fewer items are counted than are recorded: Record the missing amount as a negative value in the loss/adjustment column of batch and inventory control cards with an explanation of the reason in the remarks column. In addition a loss report should be filled (see loss report).
Corrected balances should be entered on a separate line in all related cards such as batch card, inventory stock card, and/or stock ledger, below the old balance, and a note should be written with responsible staff signature beside it, to indicate that a physical check has confirmed the new balance. This corrected total should be used for all future stock calculations.
PIC/S: The Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme (jointly referred to as PIC/S) are two international instruments between countries and pharmaceutical inspection authorities, which provide together an active and constructive co-operation in the field of GMP.
PIC/S' mission is “to lead the international development, implementation and maintenance of harmonised Good Manufacturing Practice (GMP) standards and quality systems of inspectorates in the field of medicinal products”. This is to be achieved by developing and promoting harmonized GMP standards and guidance documents; training competent authorities, in particular inspectors; assessing (and reassessing) inspectorates; and facilitating the co-operation and networking for competent authorities and international organizations.
There are currently 46 Participating Authorities in PIC/S (Convention and Scheme taken together). Participating authorities in PIC/S
The PIC (Pharmaceutical Inspection Convention) was founded in October '70 by the European Free Trade Association (EFTA), under the title of “The Convention for the Mutual Recognition of Inspections in Respect of the Manufacture of Pharmaceutical Products”. The initial members comprised the 10 member countries of EFTA at that time. In the early 1990s it was realized that because of an incompatibility between the Convention and European law, it was not possible for new countries to be admitted as members of PIC. European law did not permit individual EU countries that were members of PIC to sign agreements with other countries seeking to join PIC. As a consequence the Pharmaceutical Inspection Co-operation Scheme was formed on 2 November 1995. The Pharmaceutical Inspection Co-operation Scheme is an informal agreement between health authorities instead of a formal treaty between countries. PIC and the PIC Scheme, which operate together in parallel, are jointly referred to as PIC/S. PIC/S became operational in November 1995.
Pictogram: A graphical composition that may include a symbol and other graphical elements, such as a border, background pattern or color that is intended to convey specific information.
Pilot-scale batch: A batch of an API or FPP manufactured by a procedure fully representative of and simulating that to be applied to a full production-scale batch. For example, for solid oral dosage forms, a pilot scale is generally, at a minimum, one-tenth that of a full production scale or 100,000 tablets or capsules, whichever is the larger; unless otherwise adequately justified. (WHO)
PIR (Polyisocyanurate): A thermoset plastic typically produced as a foam and used as rigid thermal insulation. PIR is typically produced as a foam and used as rigid thermal insulation mainly in palletized shippers.
BulkPak PIR series insulated shipper (Cryopak)

Placebo control: A comparator in a vaccine trial that does not include the antigen under study. In studies of monovalent vaccines this may be an inert placebo (e.g., saline solution or the vehicle of the vaccine), or an antigenically different vaccine. In combined vaccines, this may be a control arm in which the component of the vaccine being studied is lacking. (WHO)
Positive likelihood ratio: Proportion of probability of individual with the condition having a positive test to probability that an individual without disease has a positive test. Since numerator is the definition of sensitivity, and the denominator is the converse of specificity, it can also be explain as proportion of sensitivity to converse of specificity. Also see validity.
Positive predictive value: The probability that subjects with a positive diagnostic test truly have the positive condition (disease). Also see validity.
Post-marketing surveillance: A system for monitoring adverse events following licensure (WHO). Post-marketing surveillance can be passive or active and its objectives include, but are not limited to, the following:
- the identification of rare adverse reactions not detected during pre-licensure studies; and
- the identification of risk factors or pre-existing conditions that may promote reactions.
Potency: The measure of biological activity, using a suitable quantitative biological assay, based on the attribute of the product that is linked to the relevant biological properties. (WHO)
Pre-clinical evaluation of vaccine: All in vivo and in vitro testing carried out prior to the first testing of vaccines in humans. This is a prerequisite to the initiation of clinical trials and includes product characterization, proof of concept/immunogenicity studies and animal safety testing. (WHO)
Pre-clinical toxicity study: A study designed with the primary purpose of demonstrating the safety and tolerability of a candidate vaccine product. The design of the pre-clinical toxicity study should meet the criteria outlined in the section on study design to be considered supportive of the intended clinical trial. (WHO)
Pre-exposure trial: A prospective trial in a population expected to be exposed to the pathogen under study within a predefined, relatively short, period. (WHO)
Precautionary pictograms: Pictograms indicating a recommended measure that should be taken to minimize or prevent adverse effects resulting from exposure to hazardous materials. They are always in blue background color and round shape.
Examples of (mandatory) precautionary pictograms (European Union)

Precautionary statement: A phrase (and/or pictogram) that describes recommended measures that should be taken to minimize or prevent adverse effects resulting from exposure to a hazardous product, or improper storage or handling of a hazardous product. (UN)
Precipitation coefficient: A multiplicative factor in explaining how many times faster the freeze damaged aluminum adjuvanted vaccine sediments compared to its non-frozen equivalent. Precipitation coefficient in WHO prequalified aluminum adjuvanted vaccines varies between 2 and 15. See also sedimentation rate and shake test.
Precision: The precision of an analytical procedure expresses the closeness of agreement (degree of scatter) between a series of measurements obtained from multiple sampling of the same homogeneous sample under the prescribed conditions. Precision may be considered at three levels: repeatability, intermediate precision and reproducibility. Precision should be investigated using homogeneous, authentic samples. However, if it is not possible to obtain a homogeneous sample it may be investigated using artificially prepared samples or a sample solution. The precision of an analytical procedure is usually expressed as the variance, standard deviation or coefficient of variation of a series of measurements.(ICH Q2/R1)
Preliminary Hazard Analysis (PHA): A tool that is used to determine if a particular, potential agent could be a hazard – a source of harm to something of value such as the defined critical quality attributes of a product (J Vesper). Also see preliminary risk analysis (PRA).
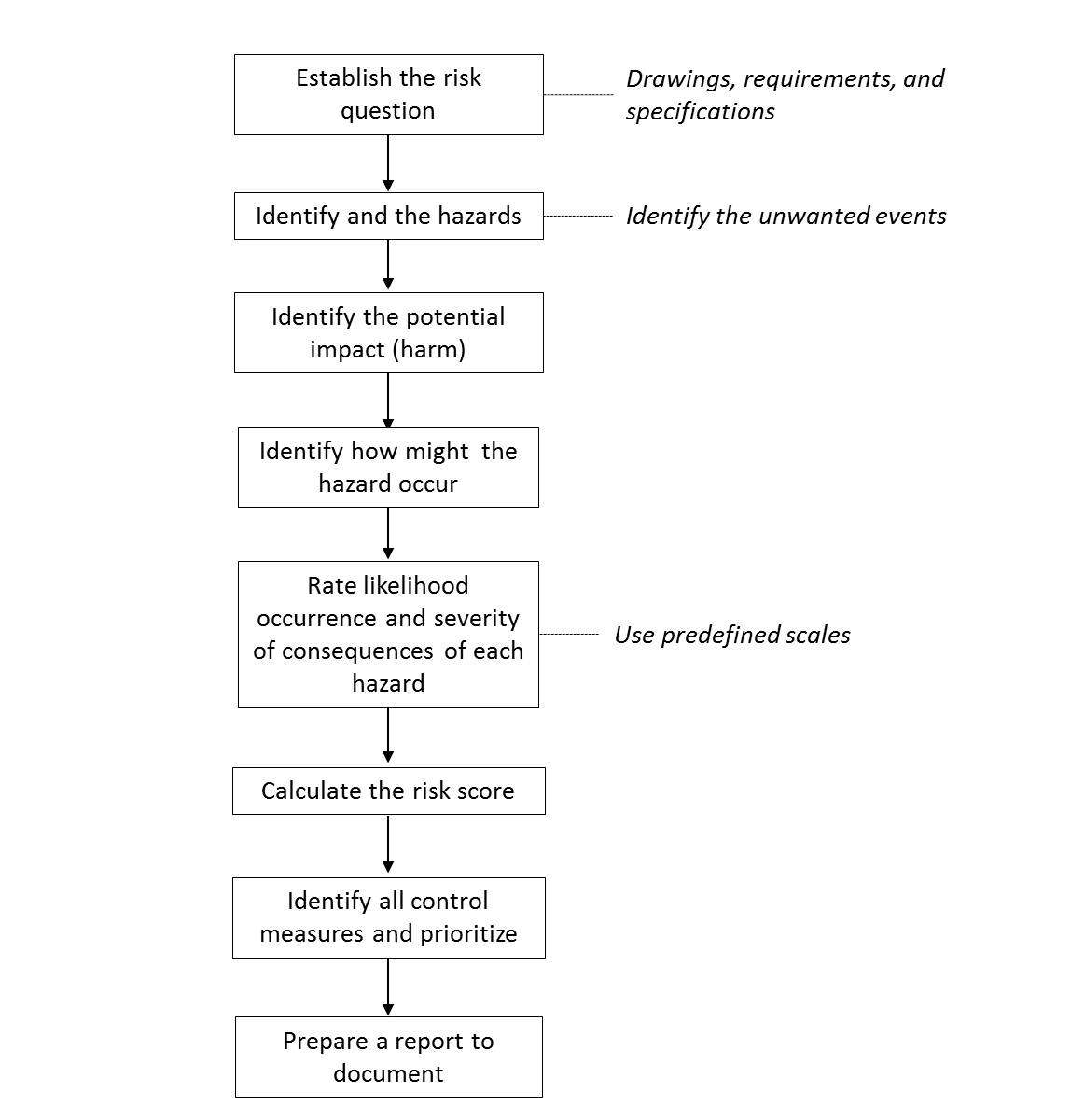
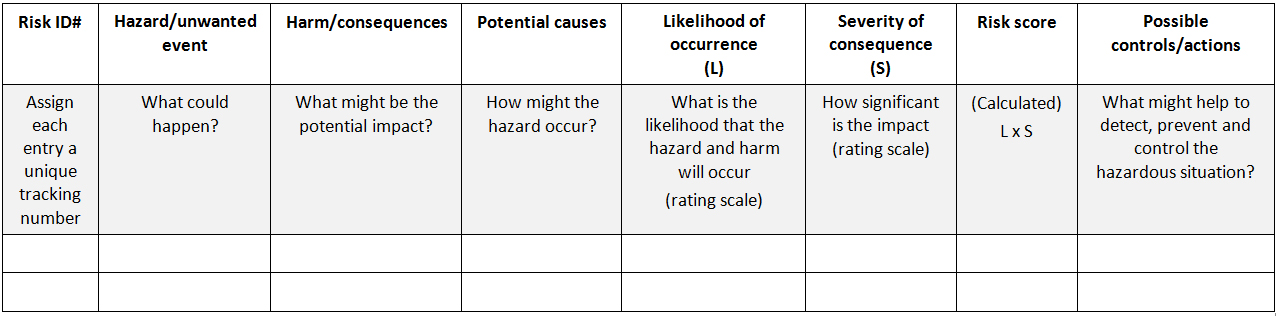
Preliminary Risk Analysis (PRA): A qualitative, inductive, loosely structured tool that can be used to identify, consider, and reduce risks early in a new or changed process. A PRA can be done at early stages of conceptual design of a product or process. Because it is relatively easy to conduct and quite unstructured, it is the recommended first step in trying to assess risks in a new product or process. Results from a PRA may lead to the use of more extensive and specific risk assessment tools for more detailed analysis. The most common approach in PRA is to study existing hazards and then examine how the hazards could be expressed, what the impact would be and the probability if occurs. On the contrary to FMEA/FMECA, detectability of the hazard is not taken into account in typical PRA/PHA.
The very first step in PRA/PHA is to establish a “risk question” that is to be studied, e.g., “What are the associated risks in using refrigerators that are equipped with non-recording temperature monitoring systems and with local alarms at the service level?” For each risk question, a separate PRA/PHA should be conducted. Under each risk question, the unwanted event is defined by asking a simple question “what could happen?” Next step is to identify the harm (consequences) and potential causes of the hazardous situation. Each hazard then is rated based on a predefined scale for its likelihood of occurrence and the severity of consequence. Multiplication of these two factors gives the “risk score” or “risk index”. Once it is completed, all possible control measures to detect, prevent, and control the hazards.
A typical PRA worksheet
Prequalified shipping container system: A packaging container or packaging system in which a DQ and OQ have already been established and documented by the manufacturer and the user has acquired sufficient documentation to meet their user requirement specification (URS).
Prevalence: The number of persons who have a particular disease at a specific time. (WHO)
Preventive action: Action to eliminate the cause of a potential nonconformity or other undesirable potential situation. Preventive action is taken to prevent occurrence.
Primary batch: A batch of an API or FPP used in a stability study, from which stability data are submitted in a registration application for the purpose of establishing a re-test period or shelf-life, as the case may be. A primary batch of an API should be at least a pilot-scale batch. For an FPP, two of the three batches should be at least pilot-scale batches, and the third batch can be smaller if it is representative with regard to the critical manufacturing steps. However, a primary batch may be a production batch.
Primary container: Bag, blister pack, strip, bottle, cartridge, vial, ampoule, prefilled device, plastic dispenser, tube, single dose container or the like containing tablet(s), capsule(s), liquid preparation or the like.
Primary vaccination: First vaccination, or series of vaccinations given within a predefined period, with an interval of less than six months between doses, to induce clinical protection. (WHO)
Primary vaccine store: A principal or main store that receives vaccine from the supplier.
Process failure: The manner in which a process fails to meet its intended purpose.
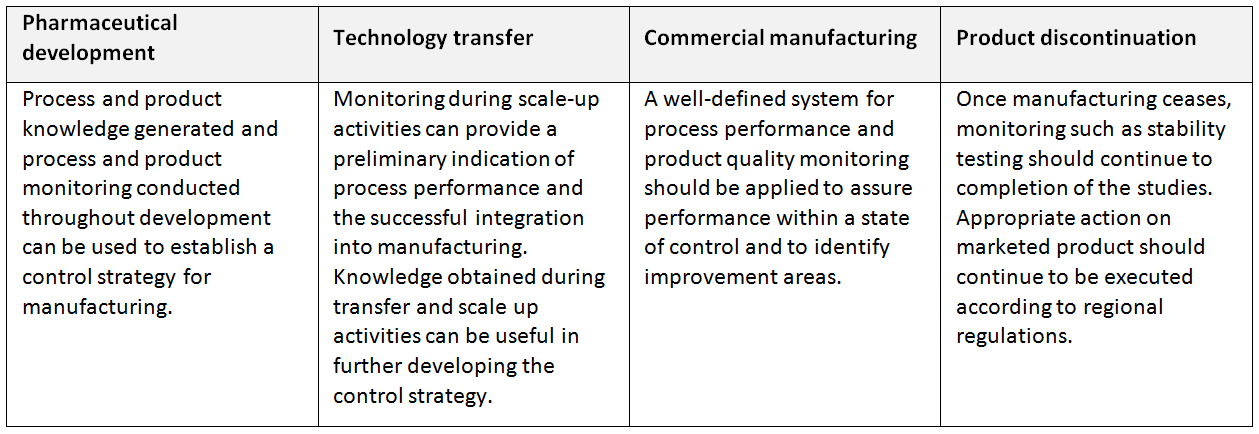
Process Performance and Product Quality Monitoring System: A system for the monitoring of process performance and product quality to ensure a state of control is maintained. An effective monitoring system provides assurance of the continued capability of processes and controls to produce a product of desired quality and to identify areas for continual improvement. As defined by the ICH Q10, the process performance and product quality monitoring system should:
- Use quality risk management to establish the control strategy. This can include parameters and attributes related to drug substance and drug product materials and components, facility and equipment operating conditions, in-process controls, finished product specifications, and the associated methods and frequency of monitoring and control. The control strategy should facilitate timely feedback / feedforward and appropriate corrective action and preventive action;
- Provide the tools for measurement and analysis of parameters and attributes identified in the control strategy (e.g., data management and statistical tools);
- Analyze parameters and attributes identified in the control strategy to verify continued operation within a state of control;
- Identify sources of variation affecting process performance and product quality for potential continual improvement activities to reduce or control variation;
- Include feedback on product quality from both internal and external sources, e.g., complaints, product rejections, non-conformances, recalls, deviations, audits and regulatory inspections and findings;
- Provide knowledge to enhance process understanding, enrich the design space (where established), and enable innovative approaches to process validation.
Application of process performance and product quality monitoring system throughout the product lifecycle (ICH Q10)
Process validation: Documented evidence that the process, operated within established parameters, can perform effectively and reproducibly to produce a drug substance or intermediate meeting its predetermined specifications and quality attributes (ICH Q7). Process validation can include the collection and evaluation of data, from the process design stage throughout production, that establish scientific evidence that a process is capable of consistently delivering a quality drug substance. As an alternative to the traditional process validation, continuous process verification (ICH Q8) can be utilised in process validation protocols for the initial commercial production and also for manufacturing process changes for the continual improvement throughout the remainder of the product lifecycle.
Product characterization: A full battery of physical, chemical and biological tests conducted for a particular product. These tests include, but are not limited to, in-process control testing, testing for adventitious agents, testing process additives and process intermediates, and lot release. (WHO)
Product realization: Achievement of a product with the quality attributes appropriate to meet the needs of patients, health care professionals, and regulatory authorities (including compliance with marketing authorisation) and internal customers’ requirements.
Product summary file: The product summary file contains general information on the company and its personnel, premises and equipment, copies of the national or regional licenses as appropriate, information on the composition of the vaccine, presentations offered, immunization schedules recommended, production methods, quality control procedures, specifications of intermediate and final products, and data on the stability, shelf-life, clinical efficacy and safety of the vaccine. For initial product assessments, a product summary file should be submitted for each vaccine to be assessed. For combination vaccines, information should be submitted on each of the component vaccines and on the combination itself.
Production batch: A batch of an API or FPP manufactured at production scale by using production equipment in a production facility as specified in the application.
Prohibitory signs: Signs prohibiting behavior likely to incur or cause danger. They are always round shape with black pictogram on white background, red edging and diagonal line.
Examples of prohibitory signs(EU)

Proportional rate: The number of cases of a particular condition as a proportion of the total number of cases of all conditions.
Protection at birth (PAB) indicator: The TT2+ indicator works well when coverage with TT is relatively low. However, as TT coverage increases, fewer women will need to receive TT (they are already protected) so the numerator will go down, but the denominator (births) will not. This will lead to an incorrect estimate of programme performance. One way to avoid this problem is by using the protection at birth indicator. This indicator measures the percentage of infants who were protected from NT at birth by the immunization of their mothers with TT before the birth.
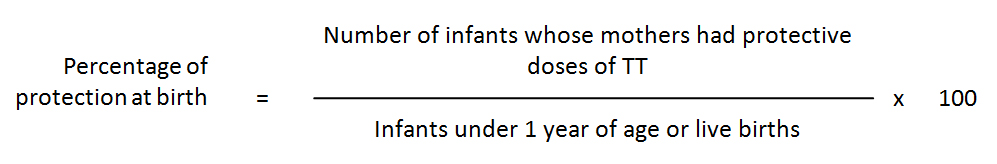
Protocol: A document that describes the objective(s), design, methodology, statistical considerations, and organization of a trial. The protocol usually also gives the background and rationale for the trial, but these could be provided in other protocol referenced documents.(ICH E6/R1)
Protocol amendment: A written description of a change(s) to or formal clarification of a protocol. (ICH E6/R1)
Provisional shelf-life: A provisional expiry date which is based on acceptable accelerated and available long-term data for the FPP to be marketed in the proposed container closure system.
Public private partnership: A partnership between the public sector and the private sector for the purpose of delivering a project or a service traditionally provided by the public sector (EU). Some so-called public-private partnerships could be more accurately described as public sector programmes with private sector participation (WHO). Some examples of public private partnerships for health include Roll Back Malaria, Safe Injection Global Network, and Stop TB (all of which have secretariats in WHO).
In 2013, football legend and United Nations Development Programme (UNDP) Goodwill Ambassador Didier Drogba joined the United Against Malaria, a campaign of “Roll Back Malaria” partnership

Pull system: A distribution system in which the personnel who receive the supplies determine the quantities to order. In the pull systems however, the lower level facilities are responsible for ordering supplies. Decisions on quantity of shipments are made by lower level managers or by the functionary responsible for supply. A pull system is best when; there is no supply shortage, the responsibility for programme operations is decentralized, and lower levels have sufficient management and data processing skills. Also called as “requisition” system. (WHO)
PUR: Polyurethane – A polymer composed of a chain of organic units joined by carbamate (urethane) links. A two-component mixture composed of isocyanate and polyol resin comes together at the tip of a gun, and forms an expanding foam that is injected into a mold. PUR resistance to heat flow (R-value) is around 7 for every 3 cm thickness of material. PUR boxes are extremely durable, very rigid and reusable. However, PUR is difficult to recycle.
Push system: A distribution system in which the personnel who issue the supplies determine the quantities to be issued. In the push systems, distribution decisions are made by higher-level facilities. The quantities distributed are usually based on usage and stock reports without receiving requisitions. It is better to choose a push system, if programme operations are centralized, data processing and analysis are conducted at the upper levels, the staff at lower levels do not have the management skills to direct a distribution system and the stocks are limited. Also called as “allocation” system. (WHO)
Once the system has been chosen as “push” or “pull”, then the responsibility for providing transport has to be decided. If responsibility is on supplying facility then it is called a distribution system. If receiving facility provides transportation then this is called a collection or pickup system. Therefore, combination of these systems can be designed depending on the needs of the country.
Possible system designs by decision making and transport responsibilities (WHO)
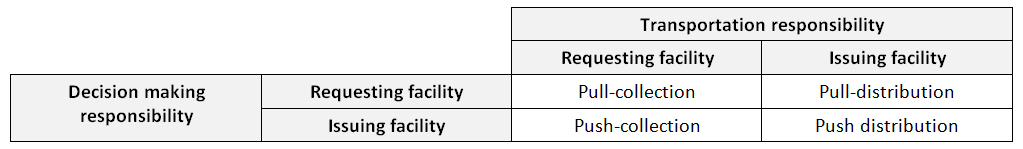
This management structure may differ at various levels of the system; higher levels may pull and then push to lower levels. Even at a single level, the system may be mixed: a regional warehouse might allocate stock to a health centre quarterly, but the health centre may be able to request additional supplies if needed.

The users of this electronic publication are free to share (to copy, distribute, display and perform the work and make derivative works based on it only for noncommercial purposes); and to remix (to adapt the work) under the following conditions:
Attribution - The work must be attributed in the manner specified by the author or licensor (but not in a way that suggests that they endorse you or your use of work)