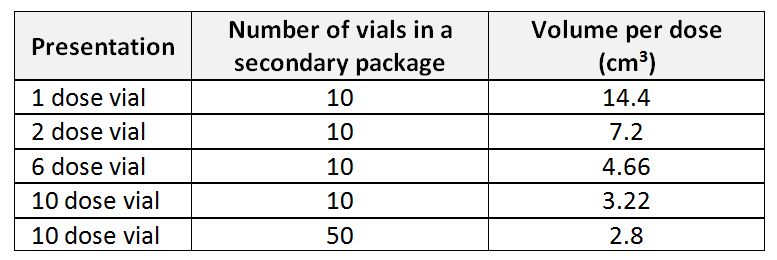V
Vaccination coverage: Percent of people who receive one or more vaccine(s) of interest in relation to the overall target population.

Vaccination failure: Vaccination failure is based on clinical endpoints or immunological criteria, where correlates or surrogate markers for disease protection exist. Vaccination failure can be due to vaccine failure (either “primary” when immune response is inadequate or “secondary” when the immune response wanes) or failure to vaccinate (i.e., when an indicated vaccine was not administered appropriately for any reason). (WHO)
Vaccine Arrival Report (VAR): The vaccine arrival report is a means of monitoring international shipments of vaccines in order to ensure that shipping guidelines are followed and that vaccine quality is maintained by encouraging increased ownership of the procurement process by all the parties involved. UN procurement agencies (UNICEF country offices, UNICEF Supply Division, PAHO Revolving Fund) enforce issuance of VARs for each shipment and for each vaccine in the shipment. Recipient governments and procurement agencies are responsible for the report, and for taking appropriate action if problems are reported (e.g., follow-up with the manufacturer, forwarding agent, WHO). For details on how to fill in the VAR, see
http://www.unicef.org/supply/files/VAR_English-with_VaxAlert_and_Qtag_instr.pdf
Vaccine arrival report used by UNICEF


Vaccine carrier: Thermally insulated box typically used to transport vaccines from health facilities with refrigeration to outreach sessions where refrigeration and ice is unavailable. They are typically carried by a single health worker travelling on foot or by other means, where the combined journey time and immunization activity lasts from a few hours to a whole day. (WHO)
Two types of vaccine carrier are described:
- Short range: With a minimum cold life of 15 hours.
- Long range: With a minimum cold life of 30 hours.
Vaccine cold box: Thermally insulated box typically used to maintain the cold chain when vaccines are transported in bulk from one fixed vaccine store to another. (WHO)
Two types of vaccine cold boxes are described:- Short range: With a minimum cold life of 48 hours.
- Long range: With a minimum cold life of 96 hours.
Vaccine effectiveness: The protection rate conferred by vaccination in a specified population. Vaccine effectiveness measures both direct and indirect protection (i.e., protection of non-vaccinated persons by the vaccinated population). Vaccine effectiveness is also determined by vaccination coverage, correlation of vaccine strains with circulating strains and incidence of disease due to strains not included in the vaccine following introduction of the vaccine in that population. (WHO)
Vaccine (protective) efficacy: The reduction in the chance or odds of developing clinical disease after vaccination relative to the chance or odds when unvaccinated (WHO). Vaccine efficacy measures direct protection (i.e., protection induced by vaccination in the vaccinated population sample). Vaccine efficacy is calculated according to the following formula:
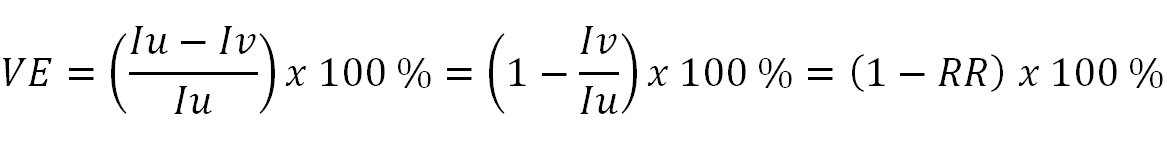
Where:
Iu = incidence in unvaccinated population; Iv = incidence in vaccinated population; RR = relative risk
Vaccine Presentation and Packaging Advisory Group (VPPAG): The VPPAG was initially set up by GAVI, the Vaccine Alliance (formerly known as the Global Alliance for Vaccines and Immunization), in 2007 to respond to specific industry requests for technical advice concerning rotavirus and pneumococcal vaccines. For the latter, VPPAG’s work fed into the World Health Organization’s (WHO) target product profile (TPP) for pneumococcal vaccines to be eligible for Advance Market Commitment (AMC) financing. VPPAG also responded to a request from industry to provide guidance on rotavirus vaccines, primarily around reducing the package volume of existing products to ease cold chain burden for resource-constrained countries. Following a successful dialogue with one company, it was possible to fruitfully engage another in a similar exercise. In 2008, WHO took over the role of convening VPPAG, and the work of the group was broadened. By 2009, VPPAG had developed a draft generic preferred product profile (gPPP) to address the range of potential new vaccines in the development pipeline. With the establishment of WHO’s Immunization Practices Advisory Committee (IPAC) in 2010, VPPAG also became a standing committee of IPAC, tasked with looking into specific issues aligned to its expertise and helping to inform IPAC’s policy recommendations that have implications for vaccine products.
Vaccine product: All components of a given vaccine formulation, including the immunogen (part of the vaccine that stimulates an immune response) and others that may be present such as the adjuvant, preservative and other additives used during the manufacturing process to confirm product quality/stability (e.g., potassium or sodium salts, albumin, gelatin), support growth and purification of specific immunogens (e.g., egg or yeast proteins, antibiotic) or inactivate toxins (e.g., formaldehyde). (WHO)
Vaccine product related reaction: An AEFI that is caused or precipitated by a vaccine due to one or more of the inherent properties of the vaccine product, whether the active component or one of the other components of the vaccine (e.g., adjuvant, preservative or stabilizer). (WHO)
Vaccine quality defect-related reaction: An AEFI that is caused or precipitated by a vaccine that is due to one or more quality defects of the vaccine product, including its administration device, as provided by the manufacturer. (WHO)
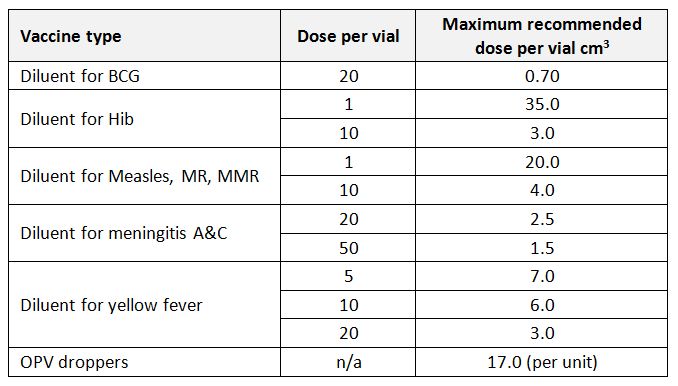
Vaccine storage capacity: For a freezer, refrigerator, cold box or vaccine carrier: the actual volume available for the storage of vaccine as stated by the equipment manufacturer or established by physical measurement. (WHO)
Vaccine usage rate: Proportion of vaccine issued which is administered, expressed in formula as follows:
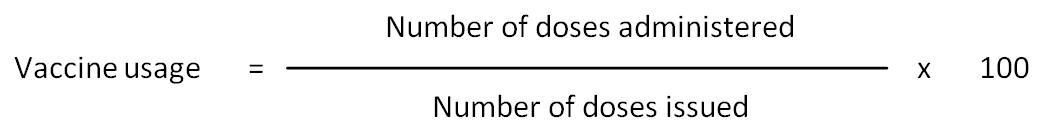
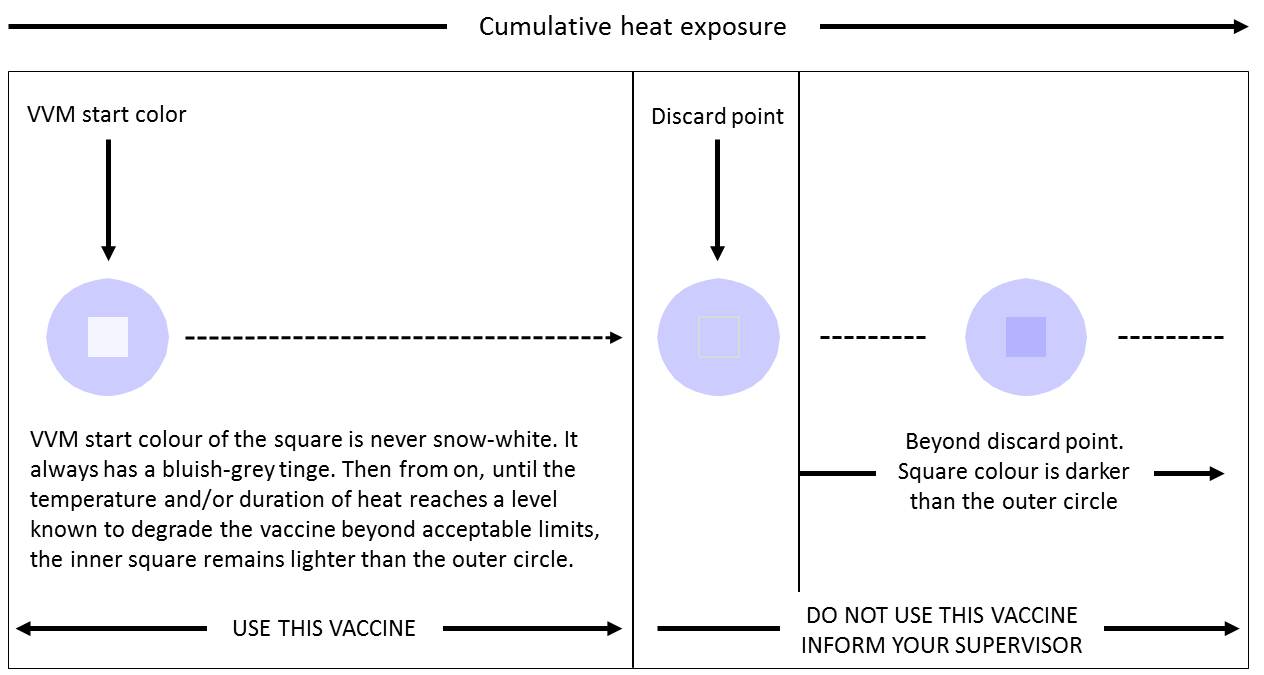
Vaccine vial monitor (VVM): A label containing a heat-sensitive material which is placed on a vaccine vial to register cumulative heat exposure over time. The combined effects of time and temperature cause the inner square of the vaccine vial monitor to darken gradually and irreversibly. A direct relationship exists between the rate of color change and temperature: the lower the temperature, the slower the color change; the higher the temperature, the faster the color change. VVM is the only tool among all time temperature indicators that is available at any time in the process of distribution and at the time a vaccine is administered indicating whether the vaccine has been exposed to a combination of excessive temperature over time and whether it is likely to have been damaged.
The principle purpose of the vaccine vial monitor (VVM) is to warn health workers when the cumulative heat exposure of a vial of vaccine has exceeded a pre-set limit, beyond which the vaccine should not be used. This is defined as the end point. Before the end point is reached, changes in the appearance of the VVM are used to alert health workers to the fact that heat exposure has occurred. Heat exposed vials can then be used in preference to those that have not been exposed. VVM is not a potency indicator. Therefore it does not directly measure vaccine potency, but gives information about the main factor that affects potency: heat exposure over a period of time.
Villagers in Indaman - a remote village in Chintabaraden region of Niger - gather for immunization (Kartoglu)

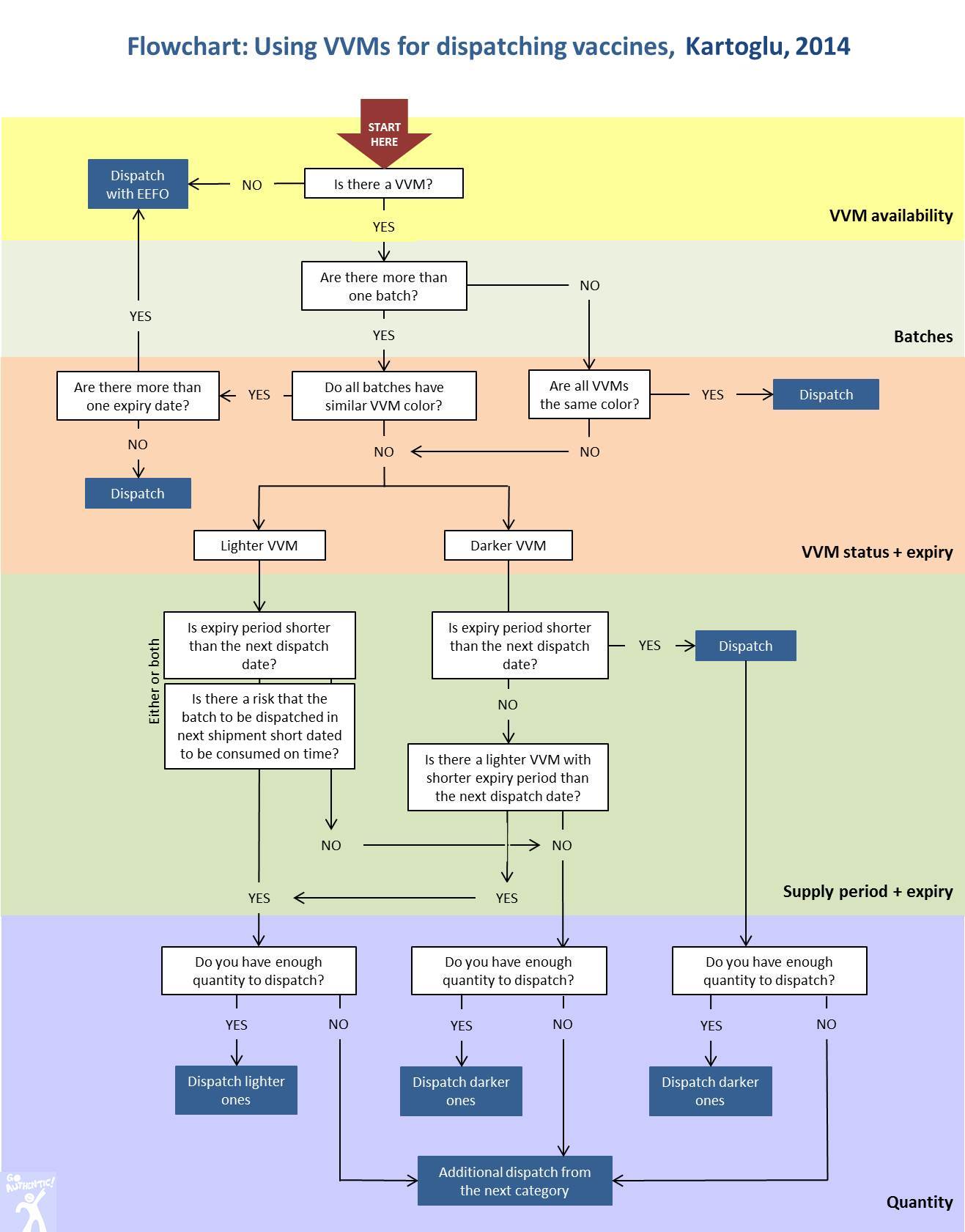
VVM reaction rates are specific to four different models of VVM, relating to four groups of vaccines according to their stability at least at two specific temperature points. Vaccine manufacturers conduct stability studies at 5oC as well as at accelerated temperatures (25oC and 37oC) to provide data for best VVM category match. Although VVM can be custom made for each product, categorization approach of stability data have shown to work well, otherwise, cost of VVM would increase.
VVM reaction rates by category of heat stability (WHO)
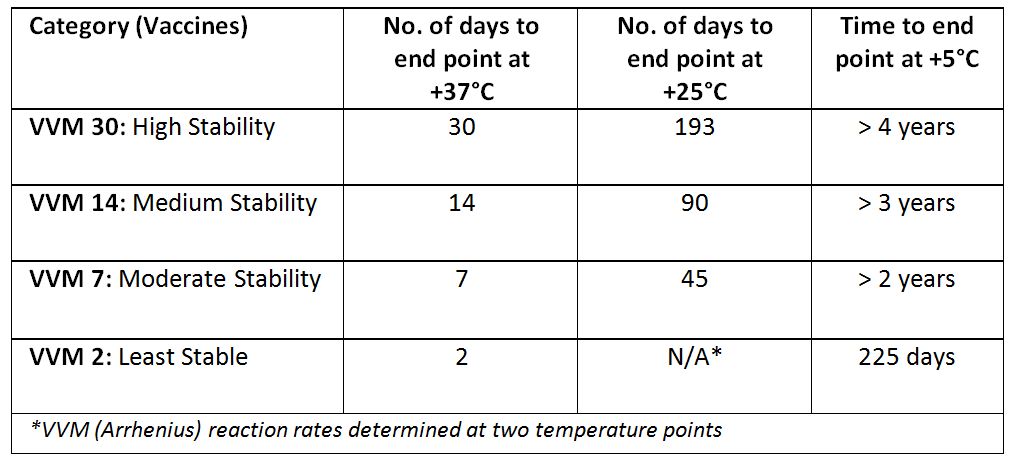
VVM reaction rates (Temptime Corp.)
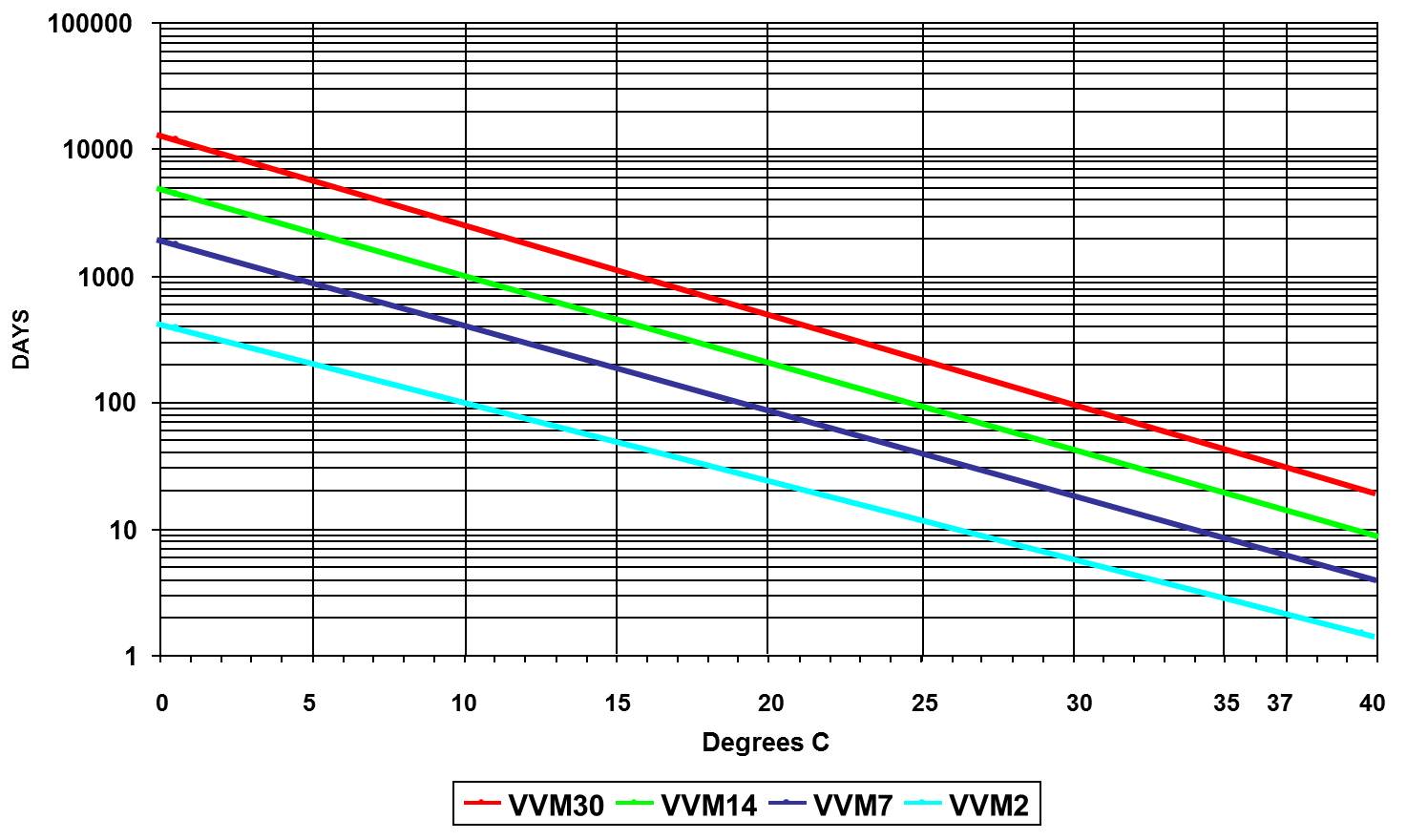
VVM performance specification and independent type-testing protocol are defined by WHO PQS protocols.
In their latest joint statement (2007) on the future of VVM, WHO and UNICEF urge Member States to include VVMs in all tender documents as well as urge donors to adopt a donation policy for inclusion of VVMs in all vaccine donations. WHO and UNICEF recommend all Member States to adopt VVM-based vaccine management policies to maximize benefits of VVM to:
- Ensure that vaccines administered have not been damaged by heat;
- Reduce vaccine wastage;
- Facilitate immunization outreach and increasing access and coverage;
- Pinpoint cold chain problems;
- Manage vaccine stocks and dispatch; and
- Prevent inadvertent freezing of vaccines.
WHO and UNICEF also recommend all Member States to consider adoption of policies permitting the use of vaccines beyond the cold chain where warranted for routine immunization activities or on a limited basis or under special circumstances, such as:
- National immunization days;
- Hard-to-reach geographical areas;
- Immunizations provided in the home – including hepatitis B vaccine birth dose;
- Cool seasons;
- Storage and transportation of freeze-sensitive vaccines where the risk of freezing is greater than the risk of heat exposure.
Vaccine wastage factor: Factor indicating how much additional vaccine should be ordered in order to allow for the given wastage rate. It is used in vaccine forecasting.
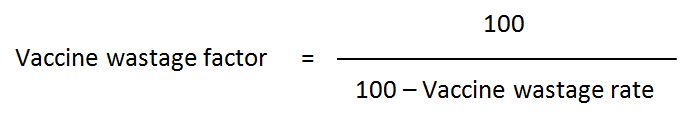
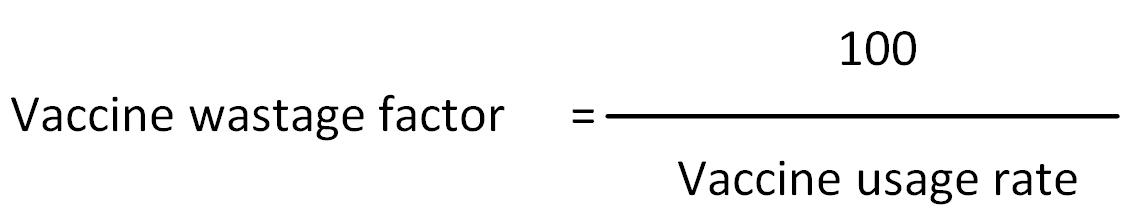
In a situation where vaccine wastage is 29%, 1.4 times more vaccine should be ordered so as to cover the estimated 29% vaccine wastage.
Vaccine wastage rates calculated from vaccine store records and service points cannot be combined since they measure and mean different things (note that the denominators of the two formulas are not the same). However, when it comes to incorporating vaccine wastage rate in estimates of national vaccine needs, both rates calculated at service level and at the storage facilities should be taken into account. National vaccine wastage rate can be calculated as follows:

For example, if the proportional vaccine wastage rate from vaccine stores is calculated to be 5% and the vaccine wastage rate from service points is 29%, the first requirement is for these to be converted into wastage factors.
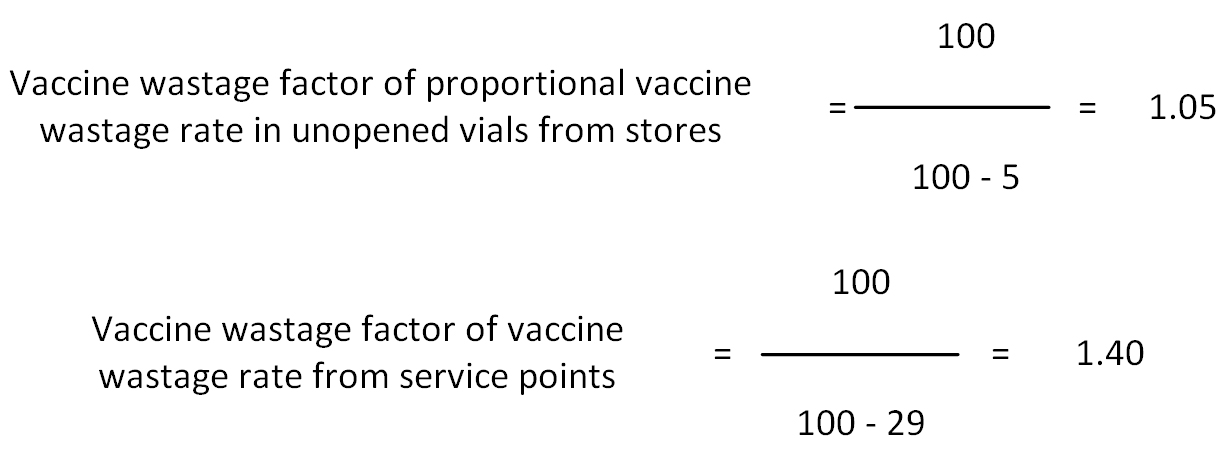
In this case the wastage factor to be included in the formula is 1.05 x 1.40 = 1.47
This means 1.47 times more vaccine should be ordered so as to cover the estimated nationwide vaccine wastage. If the wastage from storage facilities is not included in this calculation, only 1.4 times more vaccine would have been ordered instead of 1.47 times. This may result in vaccine shortage in the country.
The wastage rates cannot be merged. If they were, the total wastage rate would be 5% + 29% = 34%, giving a wastage factor of 1.51, whereas the above formula only gives a wastage factor of 1.47.
Vaccine wastage rate: Proportion of vaccine issued which is wasted (opposite of vaccine usage). Vaccine wastage happens both in opened and unopened vials. The below table summarizes the reasons for vaccine wastage:
Types of vaccine wastage (Kartoglu)
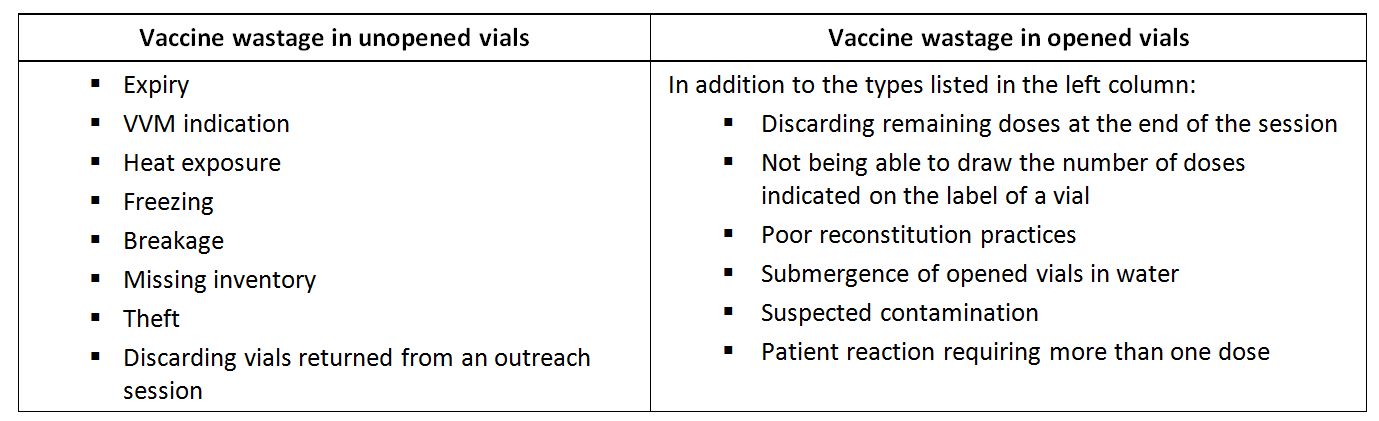
In vaccine stores, vaccine wastage occurs only in unopened vials because vaccine stores do not deliver any immunization. However, in all immunization points where immunization takes place, vaccine wastage happens both in opened and unopened vials. Therefore, vaccine wastage calculation should include both types of wastage.
Calculation of vaccine usage can be the first step in wastage calculations. Vaccine usage is defined as proportion of vaccine issued which is administered:
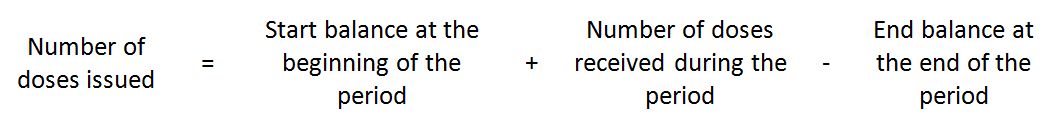
In this formula, "number of doses issued" includes doses used for immunization and all doses discarded or lost for any reason (including expiry, VVM indication, cold chain failure, freezing, missing inventory or routine discard of open vials of vaccine at the end of a session). Therefore, number of doses issued can be replaced with the following formula:
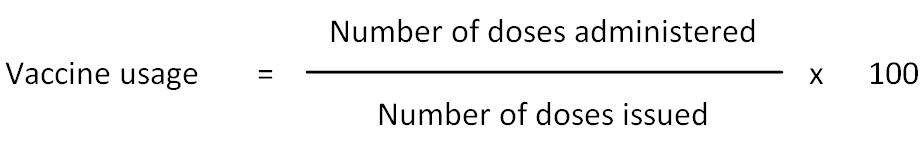
This equation which is the denominator in the formula above includes all discards in opened and unopened vials.
Vaccine wastage is the opposite of vaccine usage and is given by:
Vaccine wastage rate = 100 - vaccine usage rate
Vaccine wastage rate (in storage facilities): Because vaccine stores handle only unopened vials the above formula cannot be applied to primary and intermediate vaccine stores. For years, an erroneous practice was used to calculate vaccine wastage rates by simply using primary store figures and the number of children immunized nationwide. The best vaccine wastage indicator for vaccine stores is the proportional vaccine wastage in unopened vials. This can easily be calculated as follows.
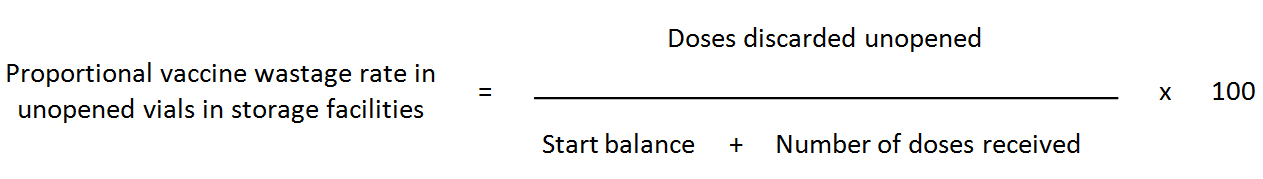
The number of doses discarded includes all discards of unopened vials because of expiry, VVM indication, heat exposure, breakage, freezing, missing inventory and theft. This rate, which is specific for vaccine stores, should not be used for comparison with the vaccine wastage rate explained above. It gives the management performance levels of vaccine stores, since these only handle unopened vaccine vials. Because this category of wastage can be minimized the question arises as to what is the acceptable level for such failures.
Vaccines delivered during the calculation period should not be subtracted from the denominator because, if any quantities of vaccine are damaged during transportation, this wastage is recorded in the sender’s vaccine store account.
Vaccines: A heterogeneous class of medicinal products containing immunogenic substances capable of inducing specific, active and protective host immunity against infectious disease.
Validation: Documented testing performed under highly controlled conditions, demonstrating that processes, methods, and systems consistently produce results meeting predetermined acceptance criteria. (PDA)
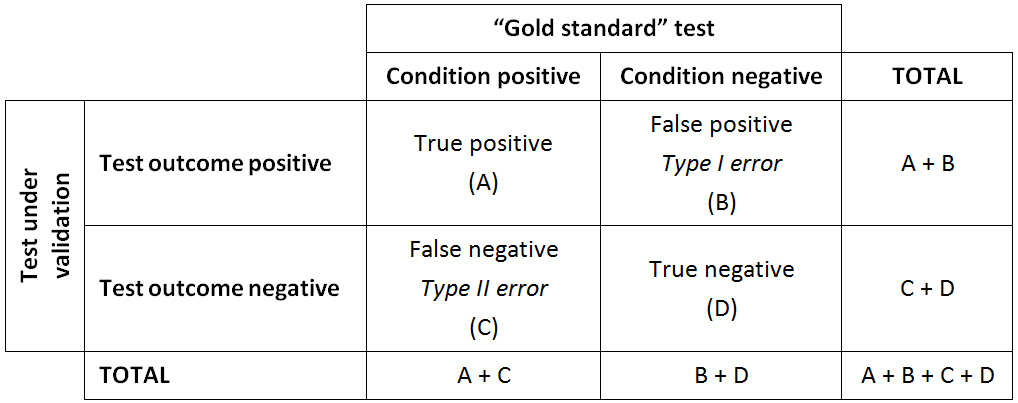
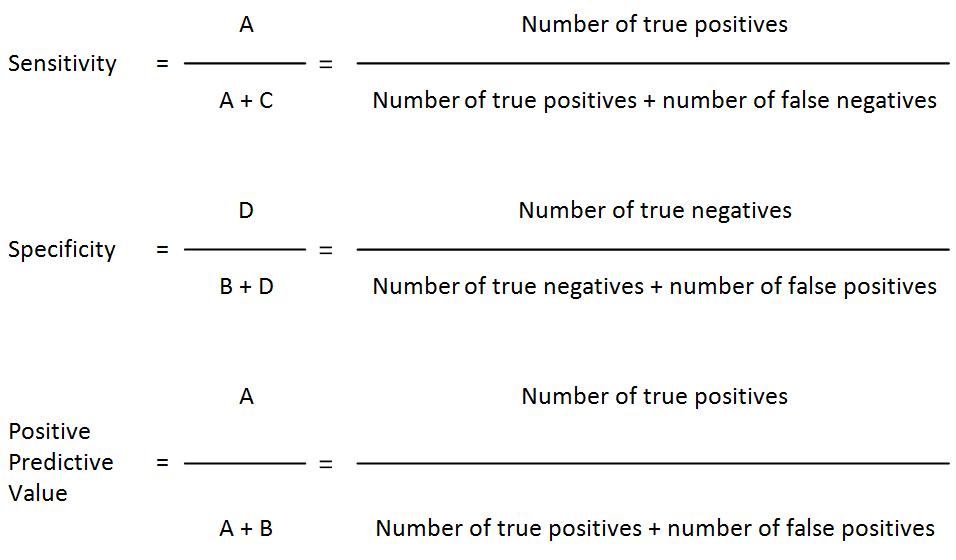
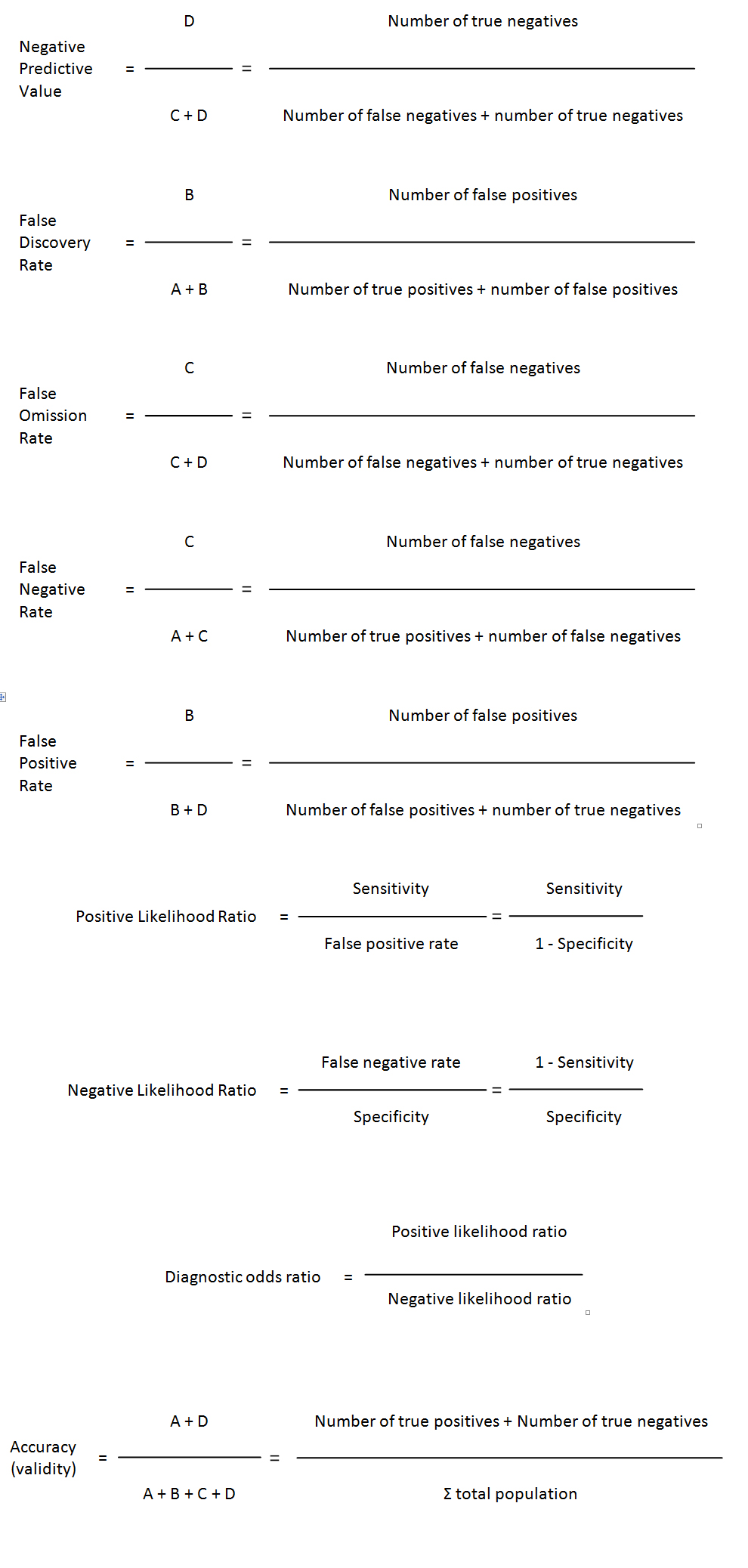
Validity (of a diagnostic test): The extent to which a test measures what it is supposed to measure; in other words, it is the accuracy of the test. Validity is measured by sensitivity and specificity. Besides sensitivity and specificity, additional rates can be calculated to explain the strength of the test under validation.
Validity rates calculations
VEN analysis: Method for categorizing stock as vital (V), essential (E), or nonessential (N). This system is sometimes modified to two categories – V and N. VEN analysis is often used to prioritize procurement when not enough funds exist to purchase all items requested. The system can also help determine which items should be kept in stock and which can be ordered when needed. (WHO) See also ABC analysis.
Vented shipping box:A container used to house an EDLM in order to record ambient air temperatures during transport, designed and constructed to maximize the airflow between the outside and inside of the container during the transport period. The container may be an integral part of a product shipment. Alternatively, if shipped separately, its overall size and weight should be similar to the container(s) used for the product(s) which are being monitored – this will ensure that the same handling practices are used. (WHO)
VIP: Vacuum insulation panels - A form of thermal insulation consisting of a nearly gas-tight enclosure surrounding a rigid core, from which the air has been evacuated. VIPs are made from nano-porous silica in a barrier film material formed under vacuum. The thermal resistance of evacuated insulation is a factor of five to ten better than conventional insulation of the same thickness.
A vacuum insulation panel (diepre, Shutterstock)

Virus: Intracellularly replicating infectious agents that are potentially pathogenic, possessing only a single type of nucleic acid (either RNA or DNA), are unable to grow and undergo binary fission, and multiply in the form of their genetic material. (ICH Q5A/R1)
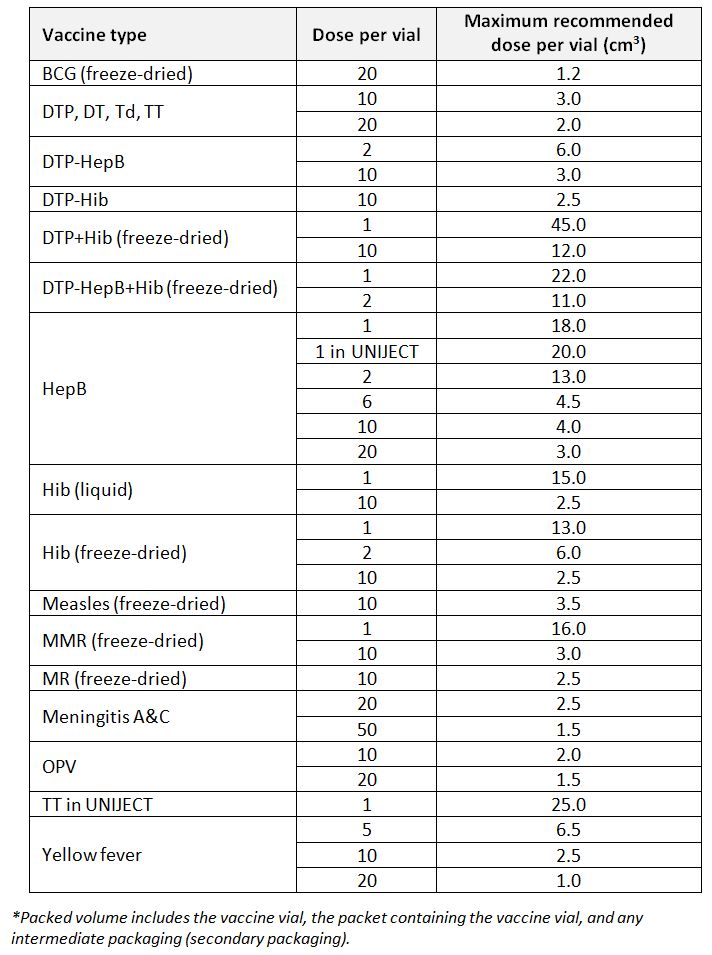
Volume per dose: The factor of volume of the secondary packaging over total number of doses of any product included in the secondary packaging. Volume per dose is an important factor for estimating required storage volume for the goods. For example, the below table provides a comparison of volume per dose for a HepB vaccine product from the same manufacturer in different vial sizes and secondary packaging:
Volume per dose variation of Hep B vaccine in various presentations and secondary packaging (WHO)