S
Safety and tolerability: The safety of a medical product concerns the medical risk to the subject, usually assessed in a clinical trial by laboratory tests (including clinical chemistry and hematology), vital signs, clinical adverse events (diseases, signs and symptoms), and other special safety tests (e.g., electrocardiograms, ophthalmology). The tolerability of the medical product represents the degree to which overt adverse effects can be tolerated by the subject. (ICH Q9)
Safety stock: The amount of stock (number of months’ supply) below the minimum level which serves as a cushion or buffer against major fluctuations in product demands or unexpected shipment delays. The safety stock is the reserve stock used to protect against stock outs due to delivery delays, product shortages at the supplier level, or when stock is dispensed at an unexpectedly high rate. The level of safety stock required is usually different for each programme and should be based on past consumption data. (WHO)
Sample size: The optimum number of subjects to be recruited to answer the main objective(s) of the study.
Seasonal packaging solution: (Also called a dedicated packaging solution). A packed shipping container system, whose effective performance in different seasons requires more than one packing configuration. These configurations depend on seasonal variants such as summer and winter or hot and cold season exposure. (WHO)
Secondary attack-rate study: An outbreak investigation in a defined susceptible population. The population to be studied is either a cluster (in an urban or semi-urban setting) or a household (or family). Outbreak investigations may be either observational or experimental. The unit of randomization may be the individual, a household or a cluster. (WHO)
Secondary pack or carton or market package: The package presentation intended for the end-user (e.g., bottle + cap liner + dose cap + leaflets + carton) but not including packaging used solely for transport purposes (e.g., Tertiary carton or Insulated shipper). The secondary pack may contain multiple units of product. (WHO)
Sedimentation rate: A measure of the particles in freeze sensitive aluminium adjuvanted vaccines sedimenting in a tube over a given period of time, expressed in percentage. The shake test is based on the difference or similarity of sedimentation rates of purposely frozen a control vial and a test vial. Frozen samples of same vaccines precipitated minimum of 2 times faster than their non-frozen equivalents. The highest precipitation coefficient was found in one of the WHO prequalified DT vaccines (15 times faster). See also precipitation coefficient and shake test.
Semi-permeable containers: Containers that allow the passage of solvent, usually water, while preventing solute loss. The mechanism for solvent transport occurs by adsorption into one container surface, diffusion through the bulk of the container material, and desorption from the other surface. Transport is driven by a partial pressure gradient. Examples of semi-permeable containers include plastic bags and semi-rigid, low-density polyethylene (LDPE) pouches for large volume parenterals (LVPs), and LDPE ampoules, bottles and vials.
Round LDPE bottle droppers

Sensitivity: Test’s ability to correctly detect patients without a condition. Also called “true negative rate”. Also see validity.
Sensor: A mechanical device (pressure switch, or bimetal temperature switch, a digital or analogue transducer (limit switch, pressure sensor, temperature sensor, etc.) that generates an electrical or mechanical signal to an instrument or a controller in order to be interpreted. (WHO)
Serious adverse event or adverse drug reaction: During clinical investigations, adverse events may occur which, if suspected to be medicinal product-related (adverse drug reactions), might be significant enough to lead to important changes in the way the medicinal product is developed (e.g., change in dose, population, needed monitoring, consent forms). This is particularly true for reactions which, in their most severe forms, threaten life or function. Such reactions should be reported promptly to regulators. (WHO)
Therefore, special medical or administrative criteria are needed to define reactions that, either due to their nature ("serious") or due to the significant, unexpected information they provide, justify expedited reporting. To ensure no confusion or misunderstanding of the difference between the terms "serious" and "severe," which are not synonymous, the following note of clarification is provided:
The term "severe" is often used to describe the intensity (severity) of a specific event (as in mild, moderate, or severe myocardial infarction); the event itself, however, may be of relatively minor medical significance (such as severe headache). This is not the same as "serious," which is based on patient/event outcome or action criteria usually associated with events that pose a threat to a patient's life or functioning. Seriousness (not severity) serves as a guide for defining regulatory reporting obligations.
After reviewing the various regulatory and other definitions in use or under discussion elsewhere, the following definition is believed to encompass the spirit and meaning of them all:
A serious adverse event (experience) or reaction is any untoward medical occurrence that at any dose:
- results in death,
- is life-threatening,
NOTE: The term "life-threatening" in the definition of "serious" refers to an event in which the patient was at risk of death at the time of the event; it does not refer to an event which hypothetically might have caused death if it were more severe.
- requires inpatient hospitalisation or prolongation of existing hospitalisation,
- results in persistent or significant disability/incapacity, or
- is a congenital anomaly/birth defect.
Medical and scientific judgement should be exercised in deciding whether expedited reporting is appropriate in other situations, such as important medical events that may not be immediately life-threatening or result in death or hospitalisation but may jeopardise the patient or may require intervention to prevent one of the other outcomes listed in the definition above. These should also usually be considered serious.
Examples of such events are intensive treatment in an emergency room or at home for allergic bronchospasm; blood dyscrasias or convulsions that do not result in hospitalisation; or development of drug dependency or drug abuse.
Serious AEFI: AEFI cases that include death, hospitalization or prolongation of existing hospitalization, persistent or significant disability or incapacity, congenital anomaly/birth defect or are life-threatening. (WHO)
Seroconversion: Predefined increase in antibody concentration, considered to correlate with the transition from seronegative to seropositive, providing information on the immunogenicity of a vaccine. If there are pre-existing antibodies, seroconversion is defined by a transition from a predefined low level to a significantly higher defined level such as a fourfold increase in geometric mean antibody concentration. (WHO)
Service delivery point: Any facility that serves clients directly and where clients (users) receive supplies. Service delivery points are frequently health posts and centres, clinics and hospitals. (WHO)
Service delivery problems: Failures identified during the analysis of the patient safety incident, which are associated with the way a service is delivered and the decisions, procedures and systems that are part of the whole process of service delivery. Service delivery problems are usually due to latent failures that arise from well-intentioned but (with hindsight) wrong management decisions that go unrecognized. (CA Vincent)
Service level agreement (SLA): A service level agreement or contract is a negotiated agreement between the customer and service provider that defines the common understanding about materials or service quality specifications, responsibilities, guarantees and communication mechanisms. It can either be legally binding, or an information agreement. The SLA may also specify the target and minimum-level performance, operation or other service attributes. (IATA)
SLA is also known as “quality agreement” and “technical agreement”.
Session size: Number of children immunized in a session. Session size is a critical factor influencing the wastage rate in opened vials. Small session size increases wastage if larger dose presentations are used. However, the opening of a new vial, even for one child, in order to avoid a missed opportunity, is always a promoted practice and should not be considered undesirable. (WHO)
Severity: A measure of the possible consequences of a hazard. (ICH Q9)
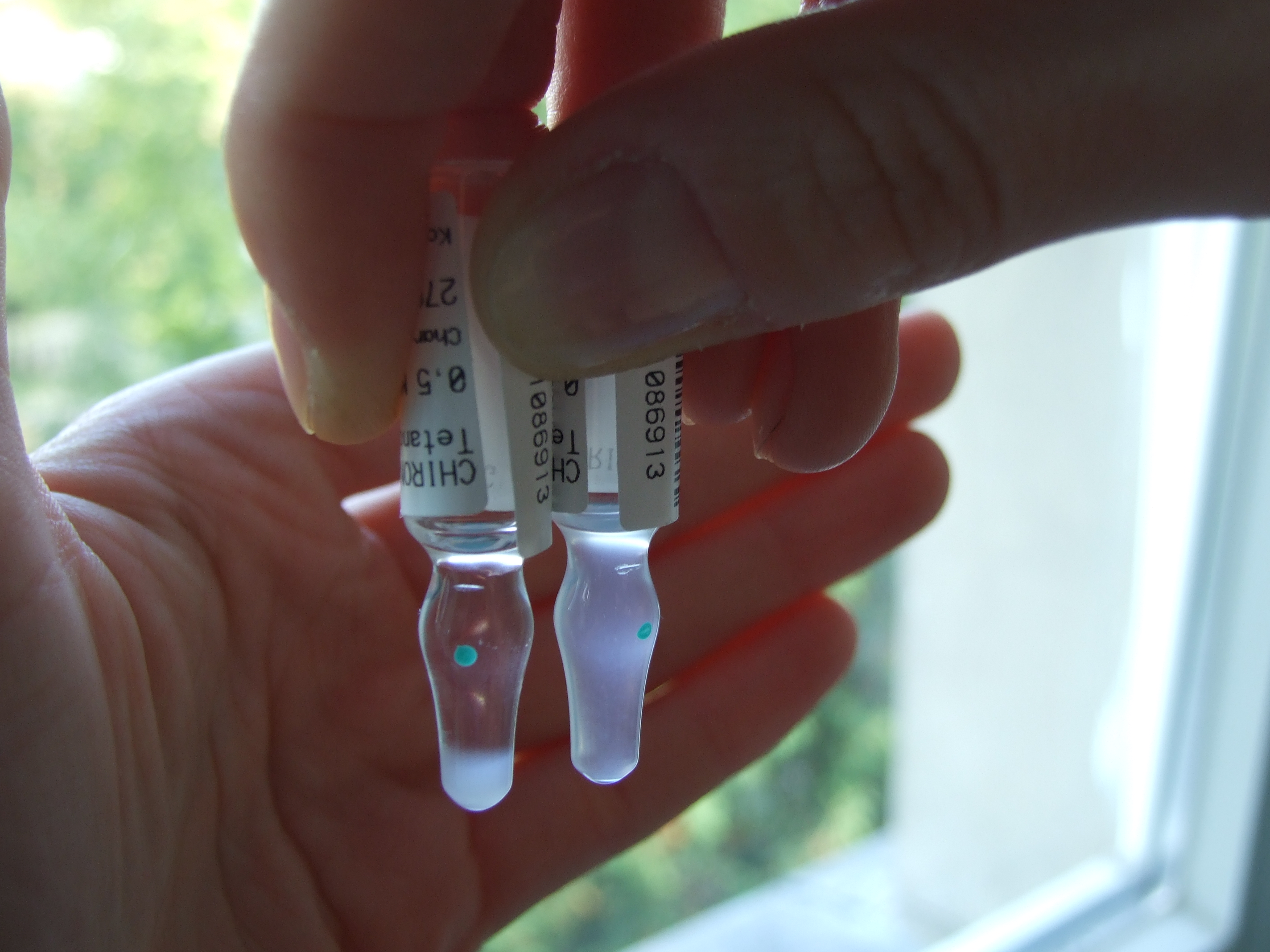
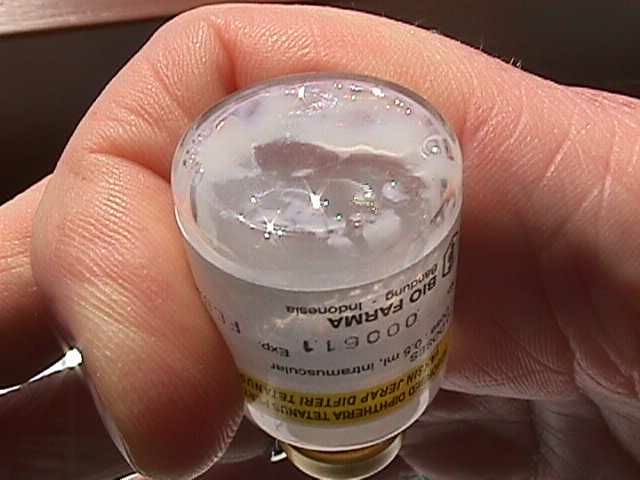
Shake test: A test that is designed to determine whether aluminium adsorbed vaccines have been damaged by freezing. Whenever it is suspected that vaccine has been exposed to freezing temperatures, at least one member of the duty personnel in every facility that stores vaccine should know how to perform and interpret the test reliably and correctly. Vaccine which fails the shake test should not be distributed or administered. (WHO)
The shake test currently applies to the following vaccines:
- DT
- DTP
- DTP-HepB
- DTP-HepB+Hib lyophilised
- DTP-HepB-Hib liquid
- DTP-Hib
- Hepatitis B
- Hib liquid
- HPV
- Pneumoccocal
- Td
- TT
After freezing, the bonds between the aluminium adsorbent and the antigen in a vaccine are broken. Separated adsorbent tends to form larger, heavier granules that gradually settle at the bottom of the vial when this is shaken. Sedimentation occurs faster in a vaccine vial which has been frozen than in a vaccine vial from the same manufacturer which has never been frozen. When carried out correctly the shake test has been shown to have 100% sensitivity and 100% specificity and 100% positive predictive value.
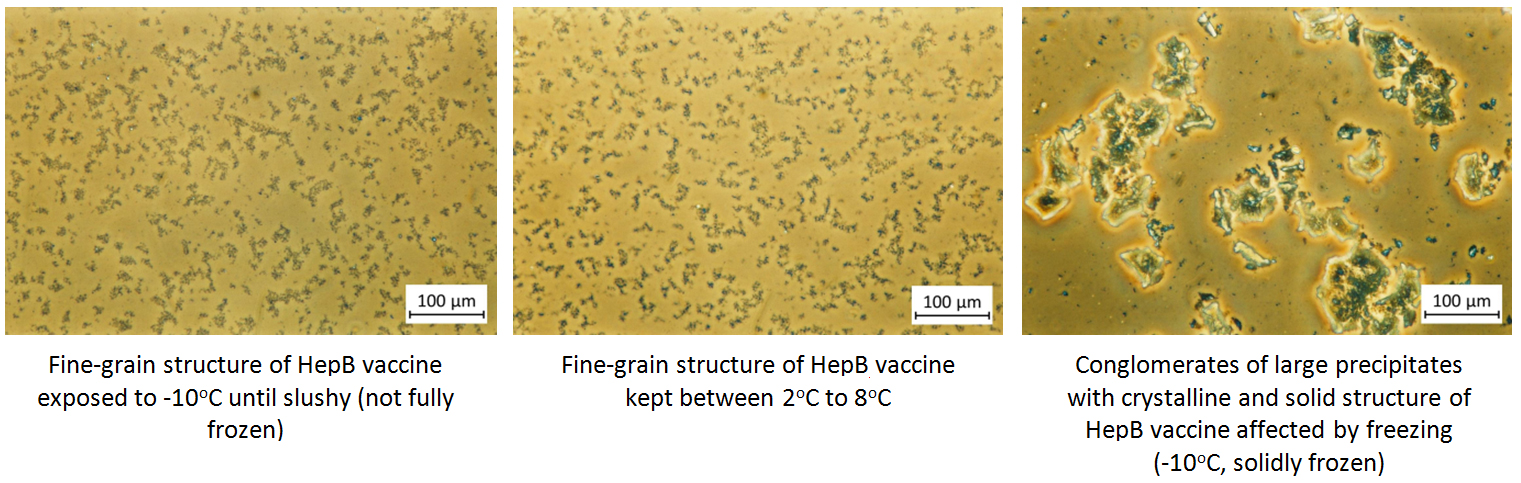
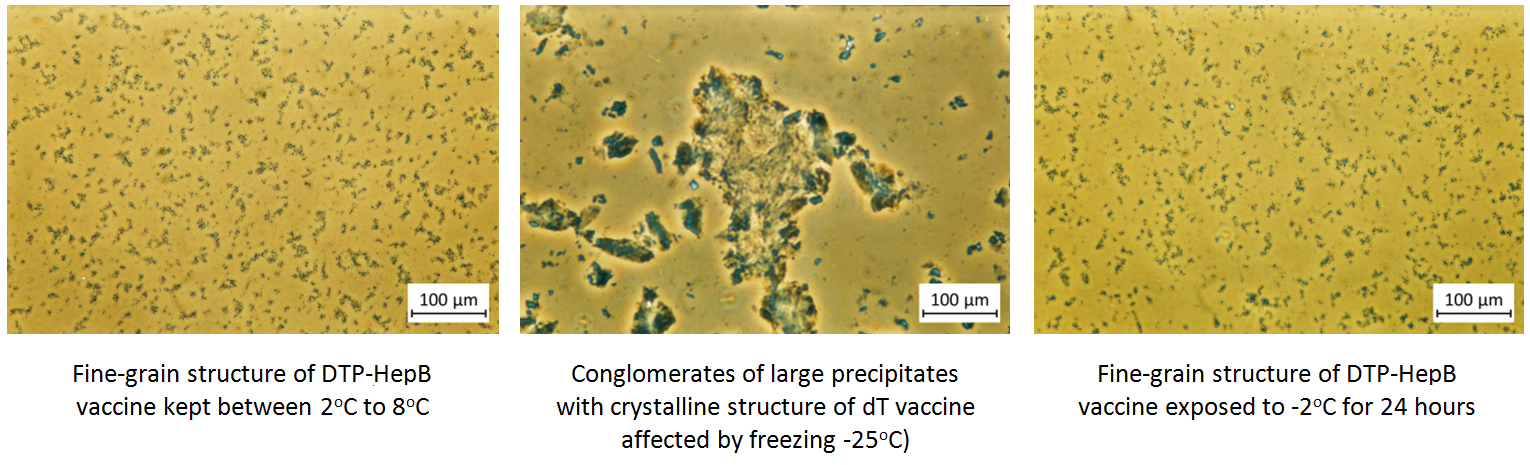
Under the phase contrast microscope, non-frozen vaccines show uniform fine grain structure while frozen vaccines show large conglomerates of large precipitates with variable structures.
In order the physical structure to be destroyed, solid freezing must take place. When vaccines are slushy frozen, their phase contrast microscopy shows identical images to vaccines that are kept at optimum temperatures. Slushy frozen vials also pass the shake test.
Phase contrast microscopy findings using study vials of various vaccines kept at different temperatures
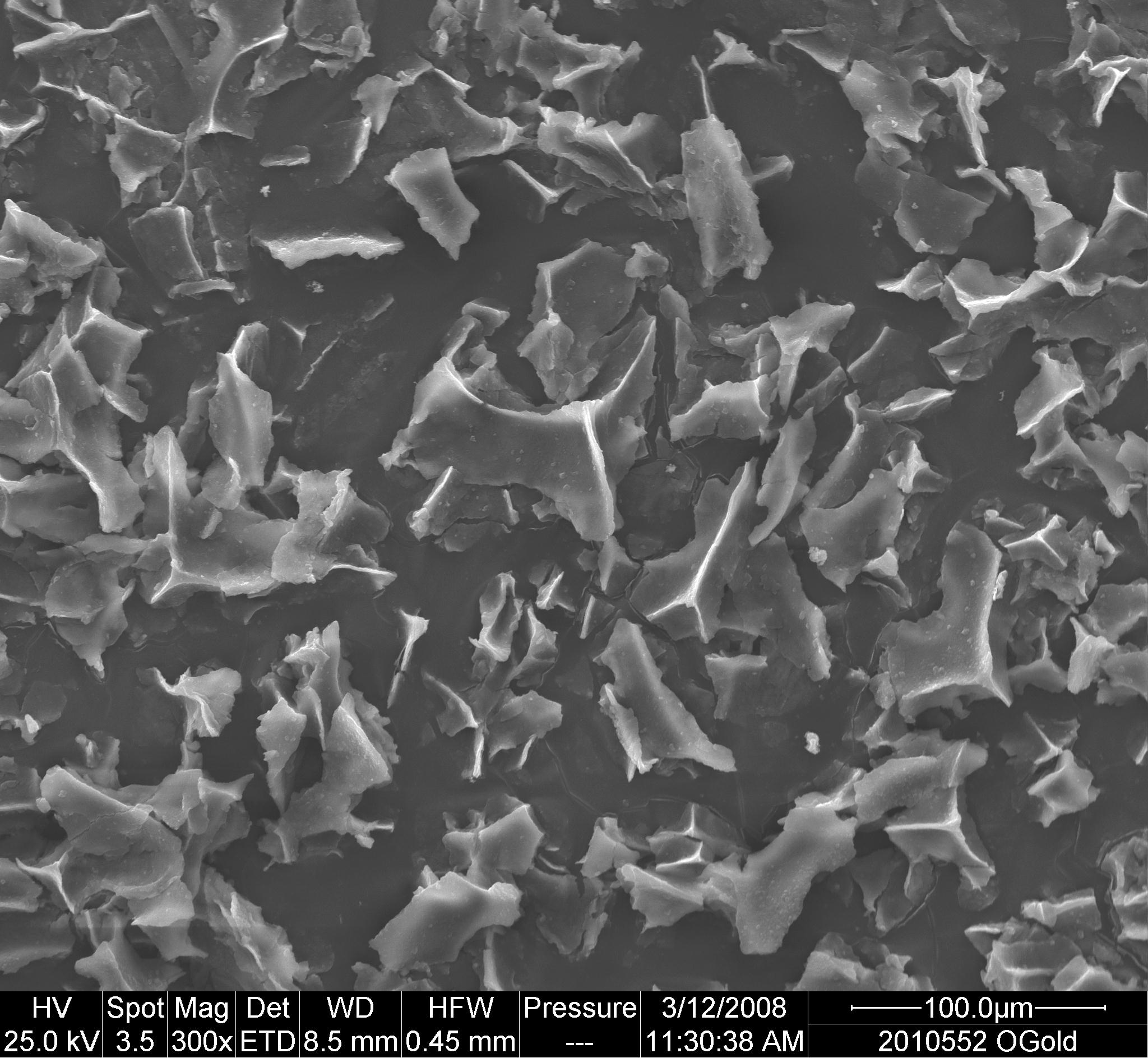
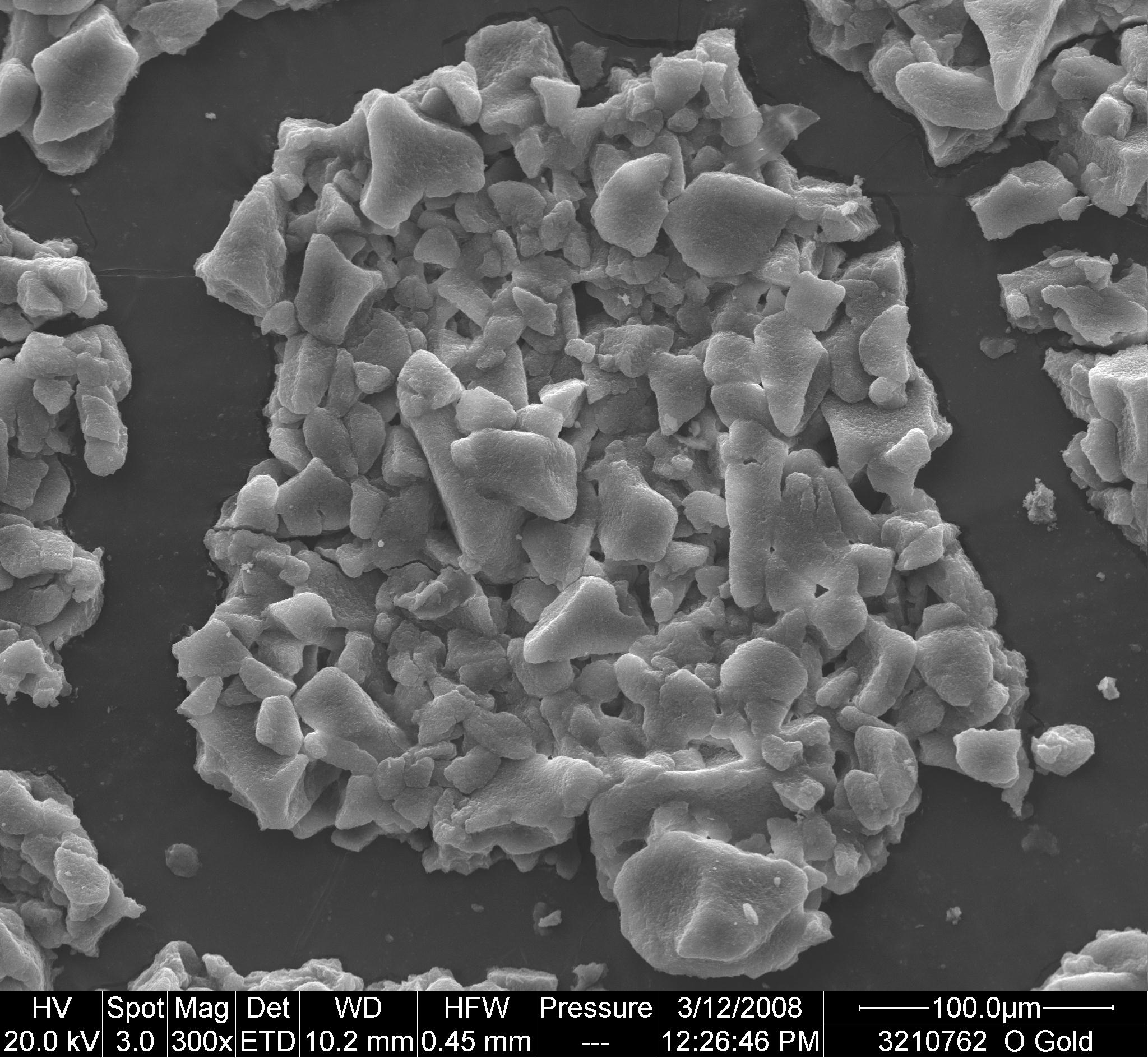
Scanning electron micrographs of gold coated conglomerates of frozen HepB, and DTP-HepB vaccines exposed to -25oC for 24 h
If a freeze indicator or other temperature monitoring device shows a freeze alarm, or if you suspect that freezing has occurred, then the shake test must be done to confirm the status of the vaccine. The shake test need not be conducted under the following circumstances; instead the vials must be discarded:
- When solid frozen vaccine vial(s) have been found.
- With a vial for which a homogeneous solution CANNOT be obtained after vigorous shaking.
In such cases, the white lumps or sediment cannot be separated from the walls of the glass vial. This happens only with DTP and its combinations vials that are exposed to sub-zero temperatures without freezing (due to P component).
Individual batches of vaccine may behave differently from one another. Therefore the test procedure described below should be repeated with all suspect batches.
The method for selecting the test sample depends upon the number of vials you suspect have been frozen and whether or not the vaccine has already been accepted from the supplier. If there are a large number of suspect vials, for example, in a cold room or a large refrigerator, you should follow the sampling procedure described in MIL-STD-105E or, a similar sampling standard, in order to establish the extent of the problem. See MIL-STD-105E.
If the test procedure shows that the test sample has been damaged by freezing, you must notify your supervisor immediately. You should then follow the procedure set out below to ensure that all of the damaged vaccine is identified and that none of this damaged vaccine is distributed or used.
Shake test protocol
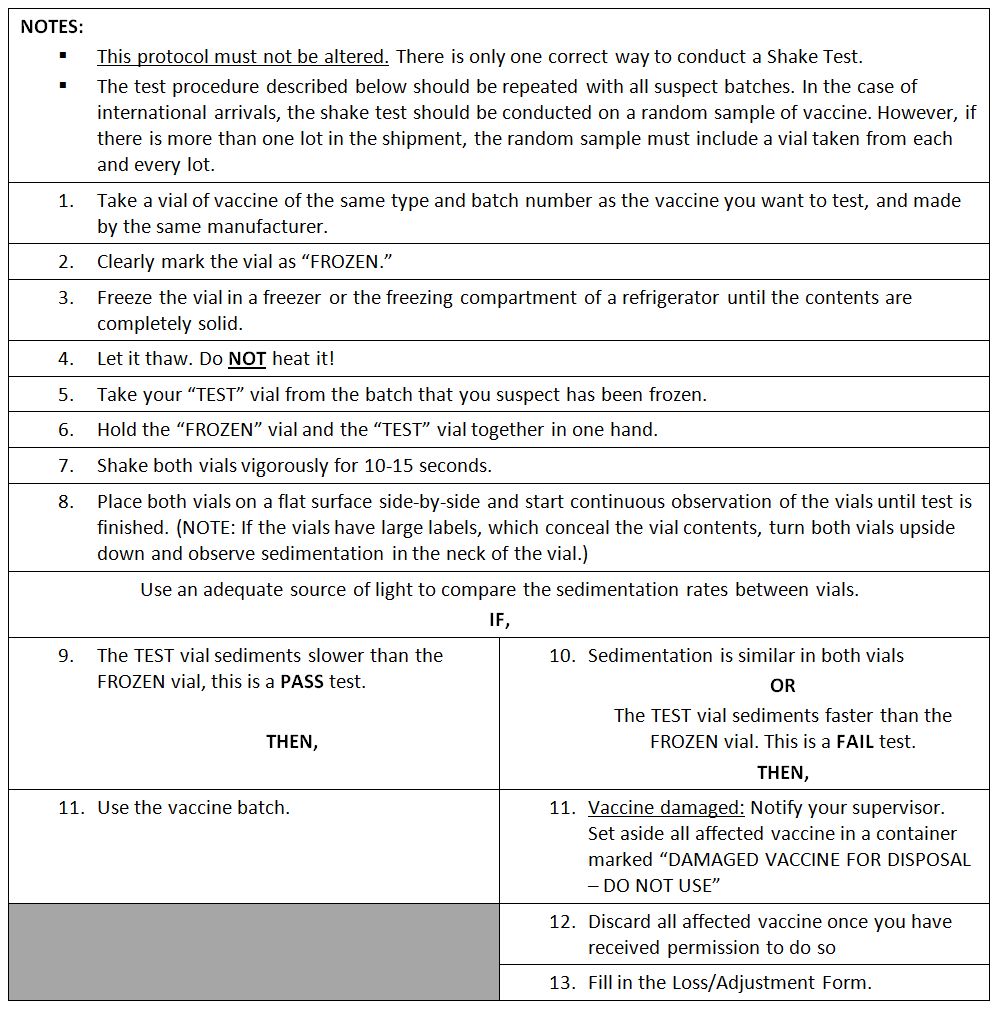
Shelf-life: The period of time during which a product, if stored correctly, is expected to comply with the specification as determined by stability studies on a number of batches of the product. The shelf-life is used to establish the expiry date of each batch. Shelf-life is used for the final product; storage period is used for the intermediates. (WHO)
Shelf-life specifications: The combination of physical, chemical, biological, and microbiological tests and acceptance criteria that an FPP should meet throughout its shelf life (should not be confused with “release specification”). (WHO)
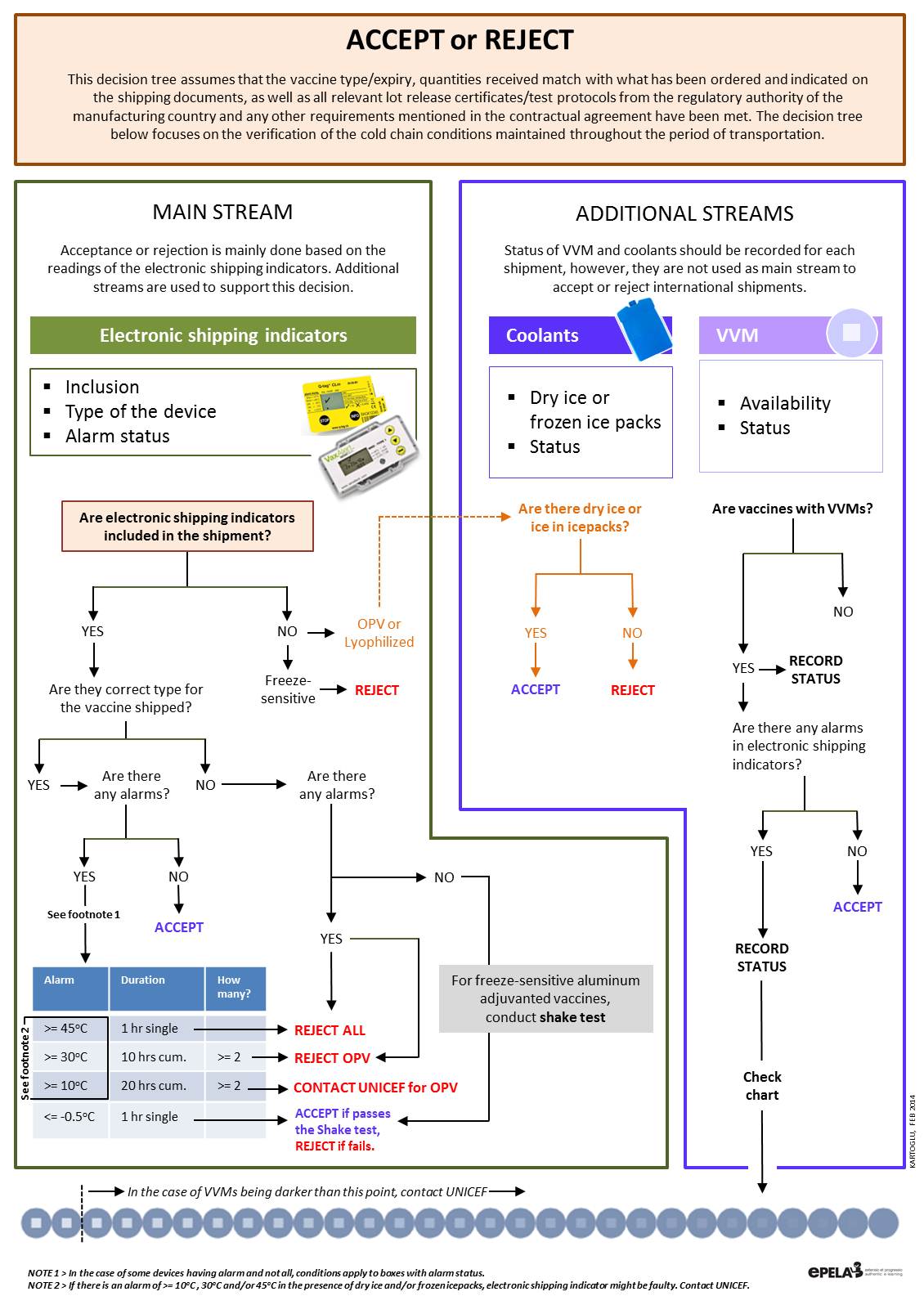
Shipping indicators: (Electronic) Shipping indicators are single-use devices designed to monitor vaccine temperature during international shipment from the manufacturer to the primary store. Although as a general category, damage indicators including threshold indicators are also considered as shipping indicators, WHO recommends use of 10 or 20 day electronic shipping indicators in each and every shipping carton. These devices serve as a quick reference to help recipient countries determine whether the shipment – or parts of the shipment – have been exposed to temperatures at which vaccines could have been damaged; and help the procurement agency determine when, where, and to what extent temperature limits have been exceeded. (WHO)
They come either with or without USB port (PDF downloadable). All 10 and 20-day electronic shipping indicators have an LCD screen for easy reference.
Various WHO PQS prequalified electronic shipping indicators


In general, shipping indicators are affixed on a waterproof backing card both to include information about the accompanied shipping as well as guidance to the receipient. There are two types of WHO recommended electronic shipping indicators to accommodate different characcteristics of vaccines.
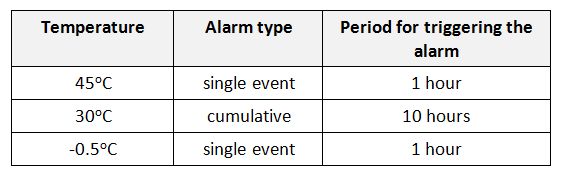
Type 1 shipping indicator is configured for freeze-sensitive vaccines and come with three pre-set alarms:
Type 1 shipping indicator alarm settings (WHO)
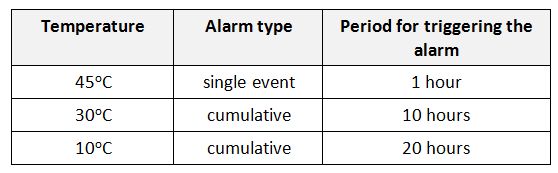
Type 2 shipping indicator is configured for lyophylized vaccines and OPV and come with three pre-set alarms:
Type 2 shipping indicator alarm settings (WHO)
As for the interpretation of electronic shipping indicators, the following flow must be followed:
Shipping lane: The route used for transporting products by different routes of transportation, including air, land, and sea. Shipping lanes originally referred to the path that sailing ships would take aided by prevailing winds and sea currents. More recently, the concept has been expanded to include transportation routes in general. Sometimes the shipping lane from the drug manufacturer to distributor to final user includes a variety of transportation modes: air cargo planes, trucks (or lorries), and even motorcycles. Knowing the shipping lane to be used – and the environmental conditions that might be encountered - helps those designing packaging select the right materials and temperature control methods. (WHO)
Shipping system: All components constituting a completed package including: the outer shipping container, all internal ancillary packaging components and temperature-stabilizing medium. (WHO)
Signal (safety signal): Information (from one or multiple sources) which suggests a new potentially causal association, or a new aspect of a known association, between an intervention and an event or set of related events, either adverse or beneficial, that is judged to be of sufficient likelihood to justify verificatory action. (CIOMS)
Significant change: In general “significant change” for an FPP is defined as the following (WHO):
- A 5% or more change in assay from its initial content of API(s), or failure to meet the acceptance criteria for potency when using biological or immunological procedures. (Note: other values may be applied, if justified, to certain products, such as multivitamins and herbal preparations.)
- Any degradation product exceeding its acceptance criterion.
- Failure to meet the acceptance criteria for appearance, physical attributes and functionality test (e.g., color, phase separation, resuspendability, caking, hardness, dose delivery per actuation). However, some changes in physical attributes (e.g., softening of suppositories, melting of creams or partial loss of adhesion for transdermal products) may be expected under accelerated conditions. Also, as appropriate for the dosage form:
- Failure to meet the acceptance criterion for pH; or
- Failure to meet the acceptance criteria for dissolution for 12 dosage units.
Six sigma: A well-structured, data-driven methodology for eliminating defects, waste, or quality control problems of all kinds in manufacturing, service delivery, management, and other business activities. Six Sigma methodology and management strategies provide an overall framework for organizing companywide quality control efforts. The term "six sigma process" comes from the notion that when there are six standard deviations between the process mean and the nearest specification limit, practically no items will fail to meet specifications.
Processes that operate with "six sigma quality" over the short term are assumed to produce long-term defect levels below 3.4 defects per million opportunities (DPMO). Six Sigma’s implicit goal is to improve all processes, but not to the 3.4 DPMO level necessarily.
Six sigma projects follow two project methodologies composed of six phases each. DMAIC (define, measure, analyze, improve, and control) is used for projects aiming to improving and existing process, while DMADV (define, measure, analyze, design, and validate) is used for projects aiming to creating new product and/or processes. DMADV methodology is also known as “design for six sigma (DFSS)”.
Normal distribution graph, which underlies the statistical assumptions of the Six Sigma model (Kartoglu)
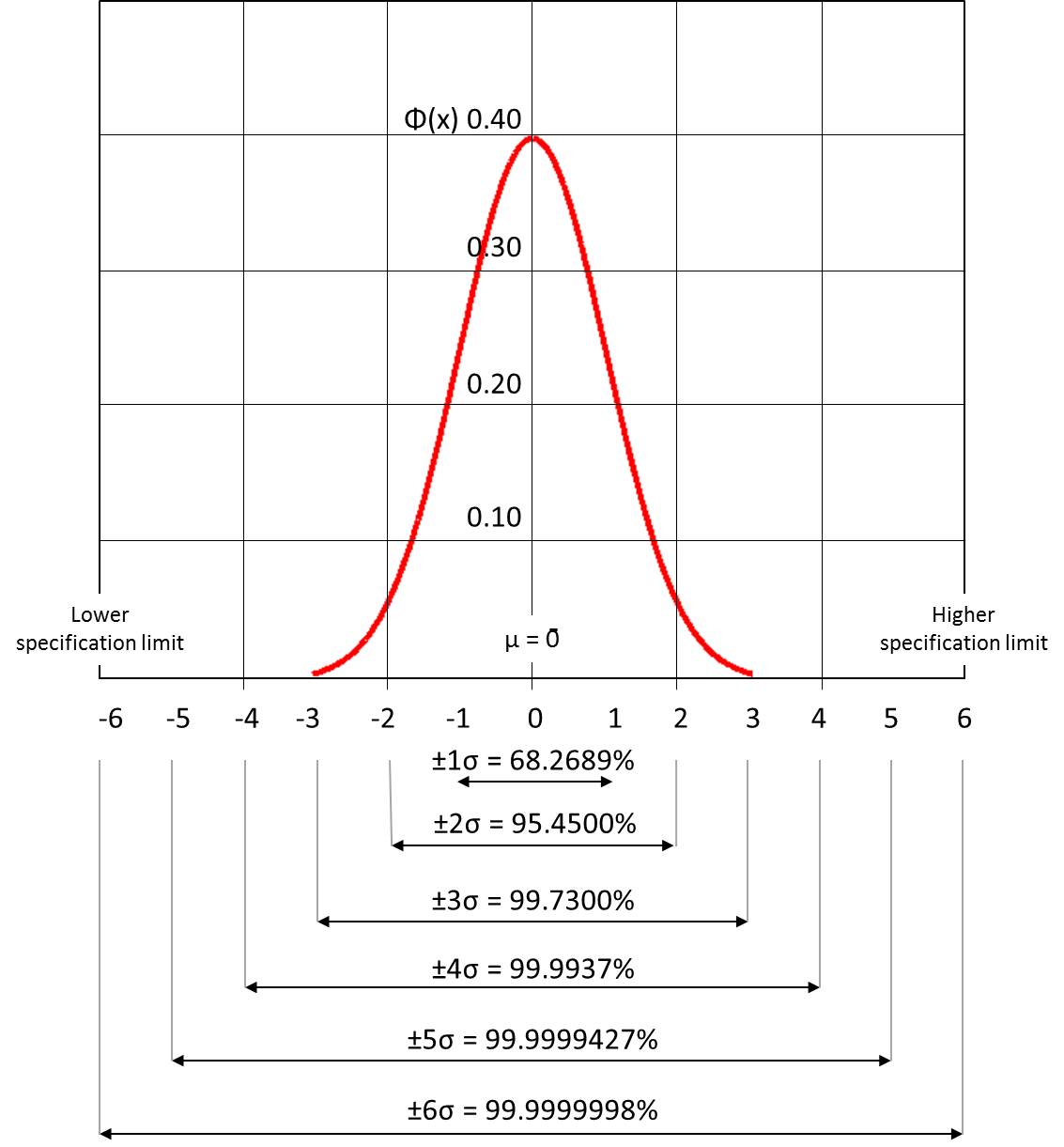
Six sigma involves absolute professionalizing of quality management functions. Formal programmes adopt a terminology similar to martial arts systems to define a special career path which includes all business functions and levels in the organization: Executive leadership, champions, master black belts, black belts, and green belts.
Solar array: A set of solar photovoltaic modules (panels) electrically connected and mounted on a supporting structure. (WHO)
Solar direct-drive refrigerator: A refrigerator that use solar energy to freeze water or other phase-change material. This stored energy is then used to provide continuous cooling, even when solar irradiance is unavailable or limited (e.g., at night or on cloudy days). (WHO)
Solar irradiance: The amount of solar energy that arrives at a specific area at a specific time. (WHO)
Solar module: A packaged, connected assembly of solar cells. Also known as a solar panel. (WHO)
Solvent: An inorganic or an organic liquid used as a vehicle for the preparation of solutions or suspensions in the synthesis of a new drug substance. (ICH Q3A/R2)
Source data: All information in original records and certified copies of original records of clinical findings, observations, or other activities in a clinical trial necessary for the reconstruction and evaluation of the trial. Source data are contained in source documents (original records or certified copies). (WHO)
Source documents: Original documents, data, and records (e.g., hospital records, clinical and office charts, laboratory notes, memoranda, subjects’ diaries or evaluation checklists, pharmacy dispensing records, recorded data from automated instruments, copies or transcriptions certified after verification as being accurate and complete, microfiches, photographic negatives, microfilm or magnetic media, x-rays, subject files, and records kept at the pharmacy, at the laboratories, and at medico-technical departments involved in the clinical trial). (WHO)
Spare parts: Parts that are available and may be used to replace or modify equipment components. (WHO)
Specific and proportional vaccine wastage rates: Specific rates reveal the types behind the overall vaccine wastage, because always unopened vial-specific vaccine wastage rate plus opened vial-specific vaccine wastage rate equals the vaccine wastage rate. (WHO)
However, unopened and opened vial proportional vaccine wastage rates do not equal the vaccine wastage rate. Proportional rates are mistakenly used by many programmes as if they reflect the “service delivery level” vaccine wastage. The denominator in proportional rates includes only the vials you calculate the proportional wastage. For example in proportional vaccine wastage rate in opened vials calculation, the denominator is doses opened for use, whereas the denominator for unopened vials proportional wastage rate is total number of doses unopened doses handled during the period. This is why; these two proportional rates cannot give the overall vaccine wastage rate.
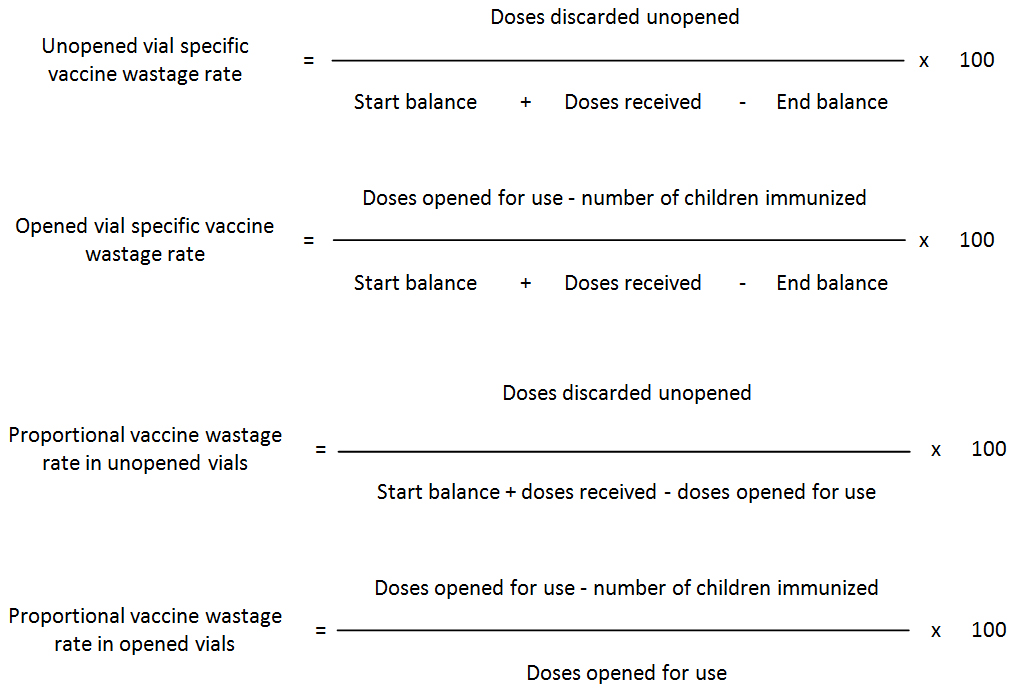
Let’s illustrate this by referring to the following example:
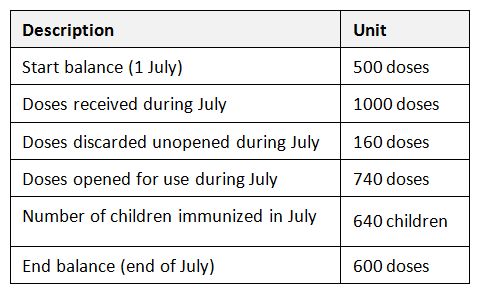
Calculation of detailed vaccine wastage at the service level
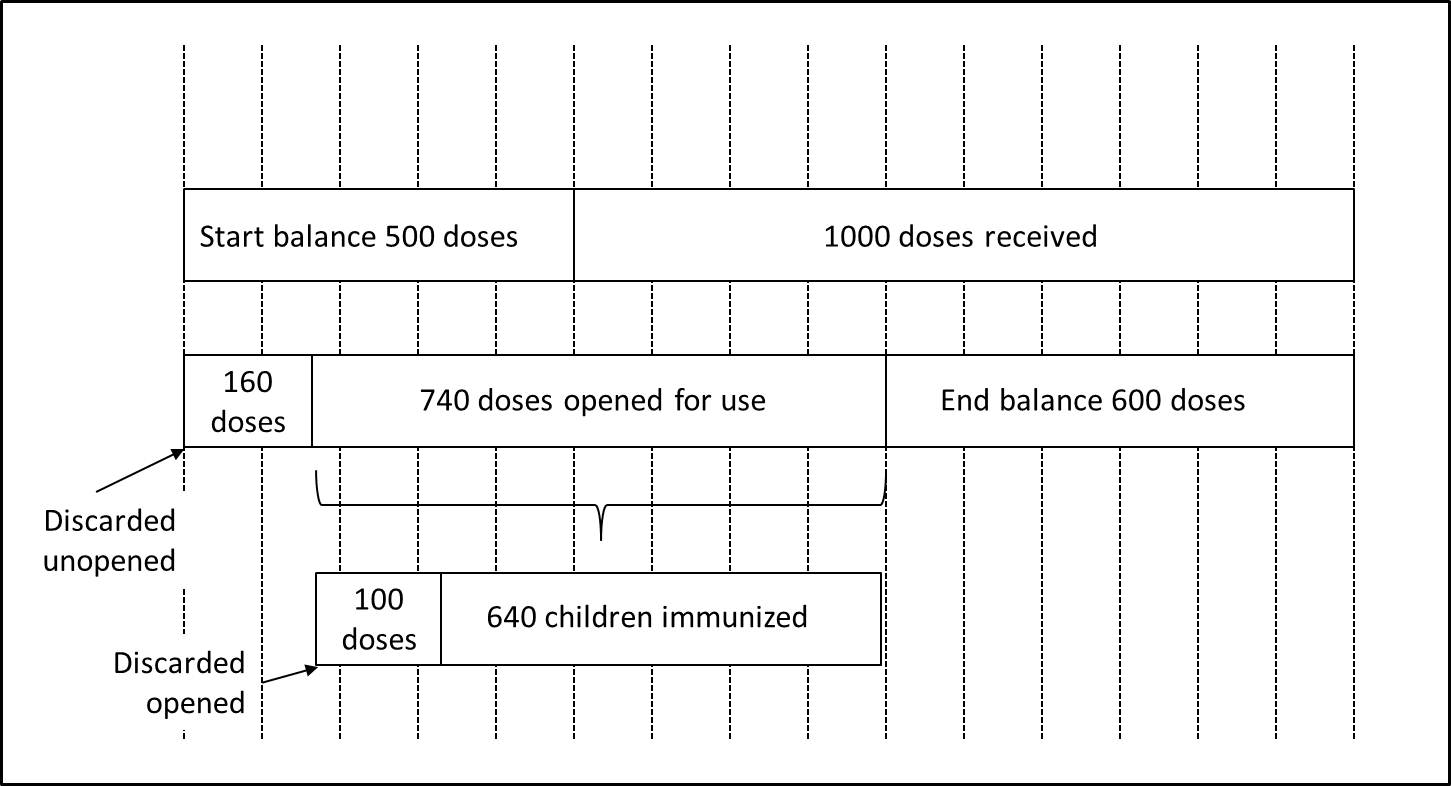
Substituting these figures in the formulas:
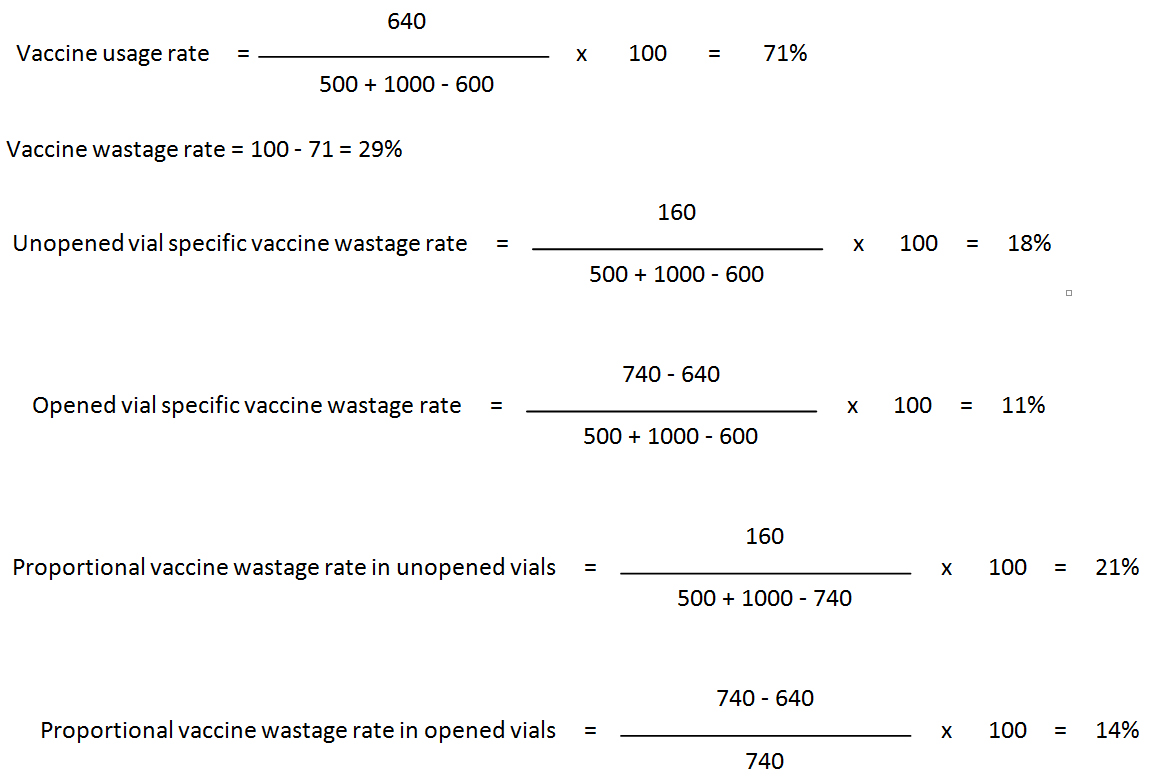
The relationship between vial specific wastage rates and overall wastage and vaccine usage (Kartoglu)
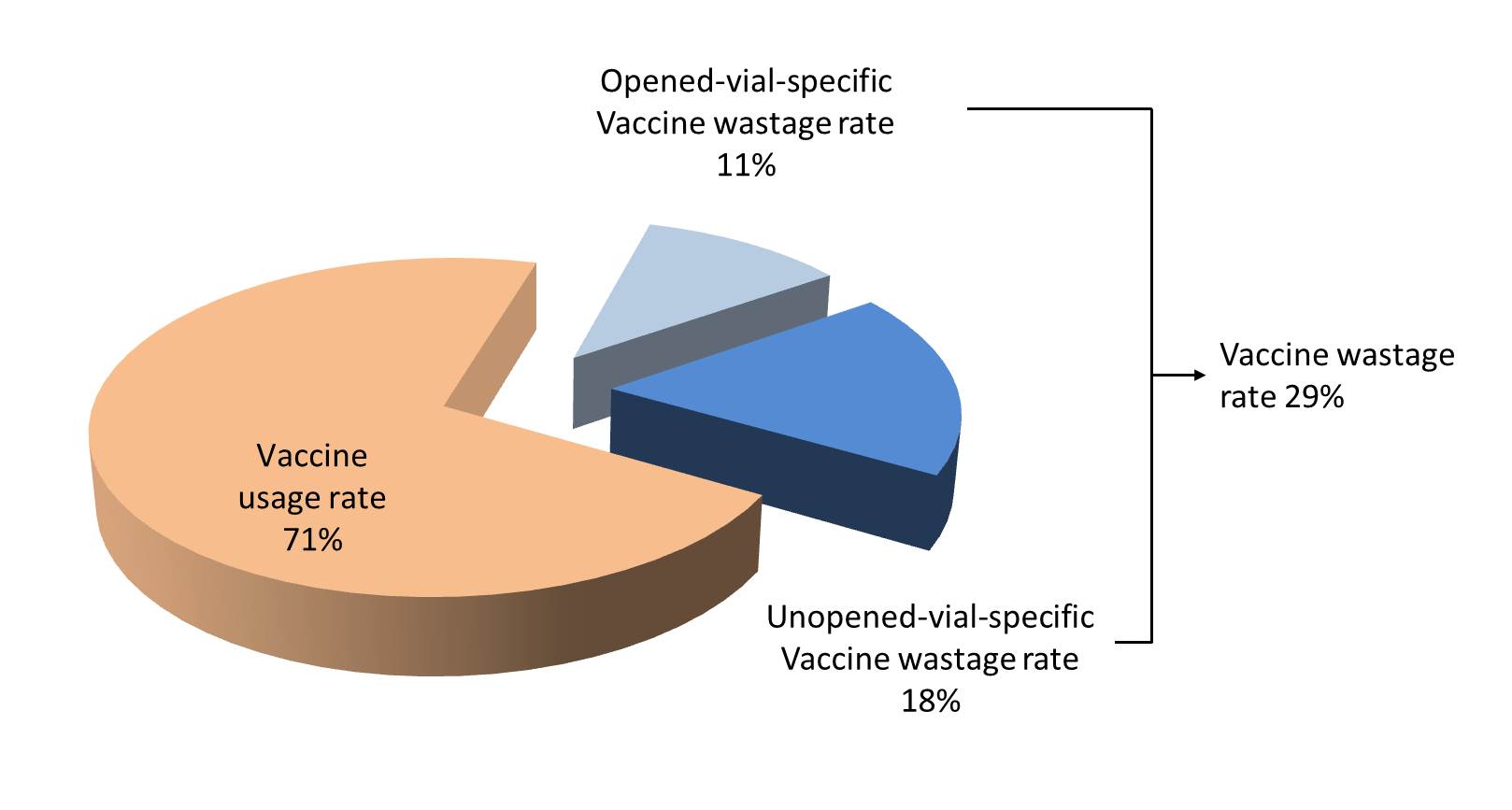
Proportional vaccine wastage rates in unopened and opened vials cannot be merged. Proportional vaccine wastage in opened vials (14%) is far below from the real overall vaccine wastage which is 29%, because this rate is proportional and does not include any other wastage occurring in unopened vials. This is why proportional wastage rates cannot and should not be used in routine monitoring of wastage rates. They are useful only in special circumstances especially in understanding the session organization success (opened vials) and stock management (unopened vials).
For further details please refer to "Kartoglu, U. Monitoring vaccine wastage at country level: Guidelines for programme managers. WHO/V&B/03.18" at http://epela.net/epela_web/document_lib/Monitoring_vaccine_wastage_at_country_level.pdf
Specific attack rate: A rate that applies to a specific demographic subgroup, e.g., individuals of specific age, sex, or race, giving the totalnumber of events in relation only to that subgroup. Specific attack rates are calculated to identify persons in the population who are at a higher risk of becoming ill than others.
Specific rate: When the numerator and the denominator of a rate are confined to a specific category (e.g., males, children under 5, Asians, etc.) it is referred to as a specific rate; e.g., age-specific death rate, or sex specific morbidity rate.
Specification: A list of tests, references to analytical procedures, and appropriate acceptance criteria which are numerical limits, ranges, or other criteria for the tests described. It establishes the set of criteria to which a drug substance or drug product should conform to be considered acceptable for its intended use. “Conformance to specifications” means that the drug substance and / or drug product, when tested according to the listed analytical procedures, will meet the listed acceptance criteria. Specifications are critical quality standards that are proposed and justified by the manufacturer and approved by regulatory authorities. (ICH Q6A)
Specificity: Test’s ability to correctly detect patients who do have a condition. Also called “true positive rate”. Also see validity.
Sponsor: An individual, company, institution, or organization which takes responsibility for the initiation, management, and/or financing of a clinical trial (ICH E6/R1). The sponsor is responsible for implementing and maintaining quality assurance and quality control systems with written SOPs to ensure that trials are conducted and data are generated, documented (recorded), and reported in compliance with the protocol, GCP, and the applicable regulatory requirement(s). (WHO)
Sponsor-investigator: An individual who both initiates and conducts, alone or with others, a clinical trial, and under whose immediate direction the investigational product is administered to, dispensed to, or used by a subject. The term does not include any person other than an individual (e.g., it does not include a corporation or an agency). The obligations of a sponsor-investigator include both those of a sponsor and those of an investigator. (ICH E6/R1)
Spurious/falsely-labelled/falsified/counterfeit (SFFC) medicines: Medicines that are deliberately and fraudulently mislabelled with respect to identity and/or source. Use of SFFC medicines may result in treatment failure or even death. Increasing international trade of pharmaceuticals and sales via the internet has further facilitated the entry of counterfeit products into the supply chain. In 2006 this led to WHO’s launch of the International Medical Products Anti-Counterfeiting Taskforce (IMPACT). Also see International Medical Products Anti-Counterfeiting Taskforce. (WHO)
Stability: The ability of a product to retain its chemical, physical, microbiological and biological properties within specified limits throughout its shelf-life. (WHO)
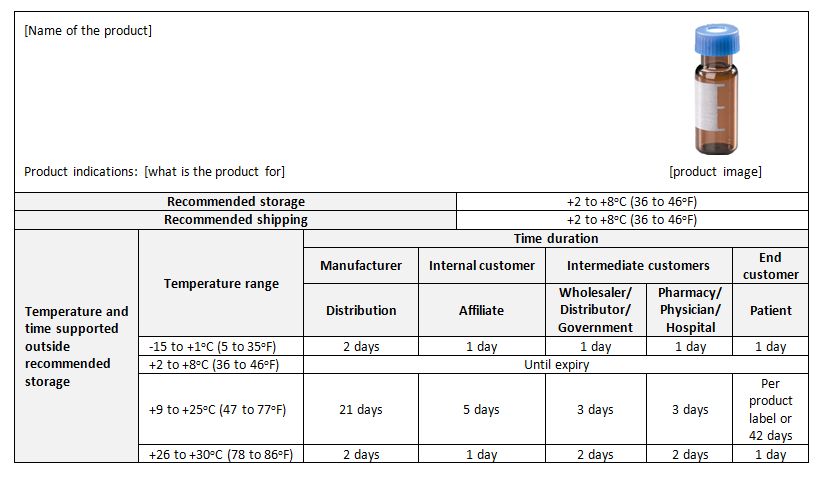
Stability budget: A stability budget considers long term, accelerated, and stress temperature exposure, as well as temperature cycling studies to determine the amount of time out of storage that a drug product may experience without any significant risk to its quality (PDA). Temperature sensitive products may have limited time that they can be exposed to temperature outside label storage conditions and still meet quality attributes through expiry. The stability budget ensures product will meet shelf life specifications given end to end time out of storage requirements.
An example of a stability budget (for an imaginary product)
Stability indicating methods: Validated analytical procedures that can detect the changes with time in the chemical, physical or microbiological properties of the API or FPP, and that are specific so that the content of the API, degradation products, and other components of interest can be accurately measured without interference. (WHO)
Stability indicating parameters: Parameters that are direct or indirect indicators of product efficacy or safety demonstrated in clinical trials. They are used to assess product suitability throughout the shelf-life. Determination of these parameters should result in quantitative values with a detectable rate of change. Qualitative parameters such as sterility could also be considered but cannot be included in the statistical analysis. (WHO)
Stability studies (stability testing): Long-term and accelerated (and intermediate) studies undertaken on primary and/or commitment batches according to a prescribed stability protocol to establish or confirm the re-test period (or shelf-life) of an API or the shelf-life of an FPP. (WHO)
Stability tests: A series of tests designed to obtain information on the stability of a product order to define its shelf-life and utilization period under specified packaging and storage conditions. (WHO)
Staging area: Zone(s) of a warehouse designated for the short-term storage of incoming goods waiting to be moved into long-term storage, and also for storing outgoing goods awaiting shipment. (WHO)
Stakeholder: A stakeholder is a person or an organization that can affect or be affected by a decision or an activity. Stakeholders also include those who have the perception that a decision or an activity can affect them. Originally it was defined in 1963 by Stanford research Institute as “those groups without whose support the organization would cease to exist”. ISO 31000 distinguishes between internal (primary) and external (secondary) stakeholders.
Standard deviation: The measure of the variability of a sample of observations around the mean.
Standard operating procedure (SOP): A set of instructions having the force of a directive, covering those features of operations that lend themselves to a definite or standardized procedure without loss of effectiveness. Standard operating policies and procedures can be effective catalysts to drive performance improvement and improve organizational results. (WHO)
Standby generator: An electrical backup system that operates automatically by sensing the power loss. When utility power returns, it switches off and returns to standby mode again. Most units run on diesel, natural gas or liquid propane gas. Standby generators should be run weekly for self-test in order to ensure proper response to outages.
Starting material: Any substance of a defined quality used in the production of a pharmaceutical product, but excluding packaging materials. (WHO)
Statute: Formal written laws enacted by the legislative authority. Statutes are also sometimes referred to as “legislation.” Some statutes provide detailed rules for addressing a particular issue. In other situations, a statute will simply set forth general principles, and the legislature will delegate the responsibility for working out the details to administrative agencies. This often is the case for issues that require a great deal of technical expertise, such as setting standards for good manufacturing practices for pharmaceutical products. Legislatures may also delegate responsibility to administrative agencies in order to avoid having to make difficult political choices. (WHO)
Stock card: A generic name for either an inventory control card or a batch card. (WHO)
Stock keeping records: Records kept on products in storage. Also see transaction records and consumption records.
Stock-keeping unit (SKU): In the field of inventory management, a code number, typically used as a machine-readable bar code, assigned to a single item of inventory. As part of a system for inventory control, the SKU represents the smallest unit of a product that can be sold from inventory, purchased, or added to inventory. Applied to wholesale, retail, or production operations, the SKU can assist in monitoring transactions, tracking customer spending patterns, controlling inventory and purchasing, and providing information about pricing, for example via its Universal Product Code (UPC). In the context of this Technical Supplement, and depending on the level in the supply chain, an SKU may be a complete pallet, a tertiary carton, a secondary carton or a primary container. (WHO)
Stock out: A condition under which there are not enough commodities on stock to meet demand. If you need to deliver 30 units and you have only 25, you are in a stock out situation. It does not necessarily mean that you have “zero” stock. However, zero stock is the “worst” case scenario of a stock out situation.
Storage conditions (of APIs): In general an API should be evaluated under storage conditions (with appropriate tolerances) that test its thermal stability and, if applicable, its sensitivity to moisture. The storage conditions and the lengths of studies chosen should be sufficient to cover storage and shipment. (WHO)
General case for storage conditions of APIs (WHO)
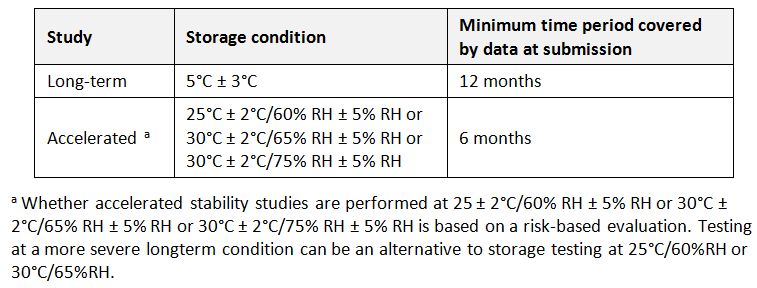

Storage temperatures: Temperatures adjusted to keep products within their recommended temperature range during storage.
Recommended vaccine storage temperatures (WHO)
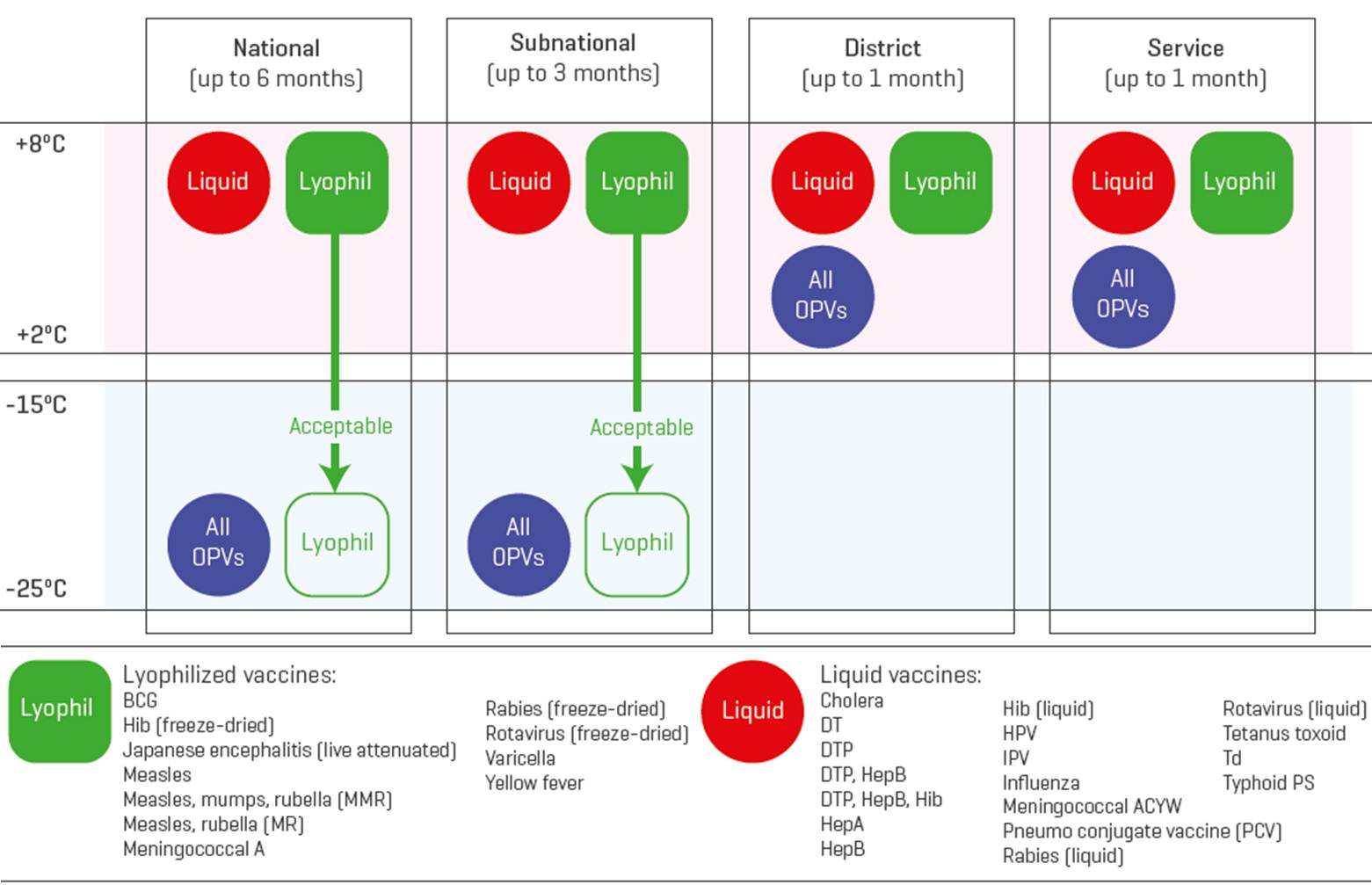
Storage unit temperature/humidity distribution: The range and pattern of temperatures and/or humidity within a temperature controlled storage unit during normal operation. (WHO)
Store ledger: A stock keeping record that keeps information about all lots of a product. Ledger format is less flexible tool for store manager, because it is easy to run out of space for an individual product. It is also hard to add new products. Individual inventory cards can be kept in ABC order, but pages cannot be alphabetized in a bound book. (WHO)
Stress testing: Studies performed to determine the impact of extreme environmental factors such as light and extreme temperature. These studies are not usually performed as part of a stability programme, but are used instead to establish protective packaging and container conditions, and to support exclusionary labelling. (WHO)
Stress testing (of the API): Studies undertaken to elucidate the intrinsic stability of API. Such testing is part of the development strategy and is normally carried out under more severe conditions than those used for accelerated testing. Stress testing of the API can help identify the likely degradation products, which, in turn, can help establish the degradation pathways and the intrinsic stability of the molecule and validate the stability-indicating power of the analytical procedures used. The nature of the stress testing will depend on the individual API and the type of FPP involved.
Stress testing may be carried out on a single batch of the API. It should include the effect of temperature (in 10°C increments (e.g., 50°C, 60°C, etc.) above the temperature used for accelerated testing), humidity (e.g., 75% relative humidity (RH) or greater) and, where appropriate, oxidation and photolysis on the API. The testing should also evaluate the susceptibility of the API to hydrolysis across a justified range of pH values when in solution or suspension. (WHO)
Stress testing (of the FPP): Studies undertaken to assess the effect of severe conditions on the FPP. Such studies include photostability testing and specific testing on certain products (e.g., metered dose inhalers, creams, emulsions, refrigerated aqueous liquid products). (WHO)
Study product: See investigational product.
Study protocol: A document detailing the scope, objectives and operational specifics of a series of tests or data collection (study) written and approved in advance of execution of the study.
Sub-investigator: Any individual member of the clinical trial team designated and supervised by the investigator at a trial site to perform critical trial-related procedures and/or to make important trial-related decisions (e.g., associates, residents, research fellows). (ICH E6/R1) See also investigator.
Subject/trial subject: An individual who participates in a clinical trial, either as a recipient of the investigational product(s) or as a control. (ICH E6/R1)
Summary report: A simple report that lists the name of the facility, reporting period, beginning quantity in hand, receipts, and quantities issued or dispensed, losses and adjustments, and ending quantity in hand for each product (usually monthly or quarterly).
A sample monthly summary report (WHO)
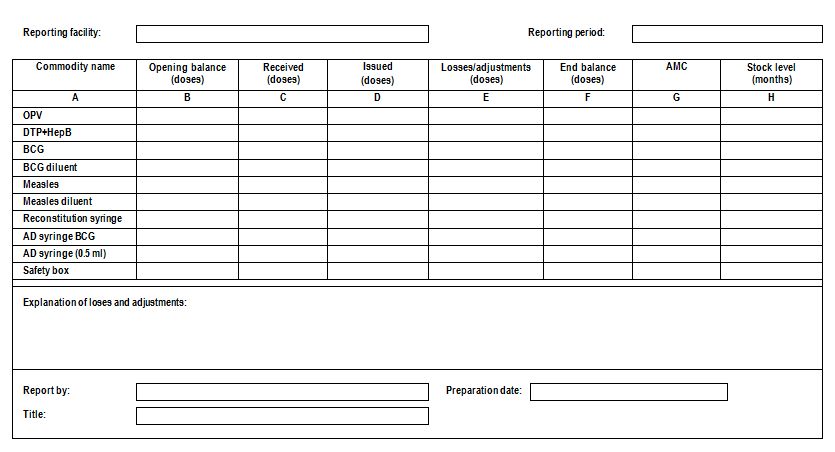
Well-designed summary reports are self-balancing. The reader can determine whether the report is mathematically correct by adding and subtracting the data on the report. Self-balancing reports are helpful because supervisors can verify the calculations. Unfortunately, self-balancing reports may not reflect actual quantities in hand if districts complete the report without comparing the end balance with the actual quantity in hand. Opening balances should be equal to the end balance of the previous report. A physical inventory conducted at the beginning or end of the month, however, may reveal a discrepancy in the beginning or ending quantity in hand. In that case, the discrepancy should be reported as a loss/adjustment for the reporting period. It is critical that the reported end balance equals the actual quantity in hand, so the quantity to be ordered will be determined directly by the actual quantity in hand and not by estimation.
Supercooling: The process of lowering the temperature of a liquid or gas below its freezing point without it becoming a solid. A liquid crossing its standard freezing point will crystalize in the presence of a seed crystal or nucleus around which a crystal structure can form creating a solid. Lacking any such nuclei, the liquid phase can be maintained all the way down to the temperature at which crystal homogeneous nucleation occurs.
The melting of a solid above the freezing point (the process opposite to supercoiling) does not work since a solid will almost always melt at the same temperature for a given pressure. Because of this, “melting point” is identified in establishing scientific freezing point. This is often called “the principle of observing the disappearance rather than formation of ice”.
Superiority trial: A trial with the primary objective of showing that the response to the investigational product is superior to a comparative agent (active or placebo control). (ICH E9)
Supply chain: A system of organizations, people, activities, information, and resources involved in moving a product from the supplier to end user in a manner that ensures that the product arrives in good condition. (WHO)
Supporting stability data: Supplementary data, such as stability data on small-scale batches, related formulations, and products presented in containers other than those proposed for marketing, and scientific rationales that support the analytical procedures, the proposed retest period or the shelf-life and storage conditions. (WHO)
Surveillance: The continuing, systematic collection of data that is analyzed and disseminated to enable decision-making and action to protect the health of populations.
Surveillance aims to assess the magnitude of problem; monitoring implantation of health programmes; understanding local epidemiology of the problem; assessing changes in disease trends or its distribution in the community; identifying specific risk groups; and assessing the impact of the intervention programme on control of diseases.
The data-collection method determines the type of surveillance, so that:
- passive surveillance is based on passive reporting;
- sentinel surveillance is based on selected institutions or people;
- active surveillance is based on systematic search for cases.
Passive surveillance is the routine reporting if diseases from health care facilities. Active surveillance, in contrast to passive surveillance, seeks to ascertain completely the number of adverse events via a continuous pre-organised process. Examples of active surveillance are, cohort studies that have been conducted in the United States of America, the United Kingdom, Denmark and others, to evaluate the safety of vaccines, in which all outcomes for specified events are identified in a predefined cohort using clinical information databases. Using such systems it is possible to ascertain events in a population completely, and in an unbiased manner, which facilitates accurate assessment of any potential safety issues. (WHO)
Suspect product: A TTSPP whose presentation and/or pharmacological formulation indicates that it has not been manufactured by the company named on the packaging. A suspect product may show visible or pharmacological evidence of tampering. (WHO)
Suspected immunization error: Information (from one or multiple sources) which suggests a new and potentially causal association, or a new aspect of a known association, between an intervention and an adverse event or set of related adverse events, that is judged to be of sufficient likelihood to justify verificatory action. (WHO)
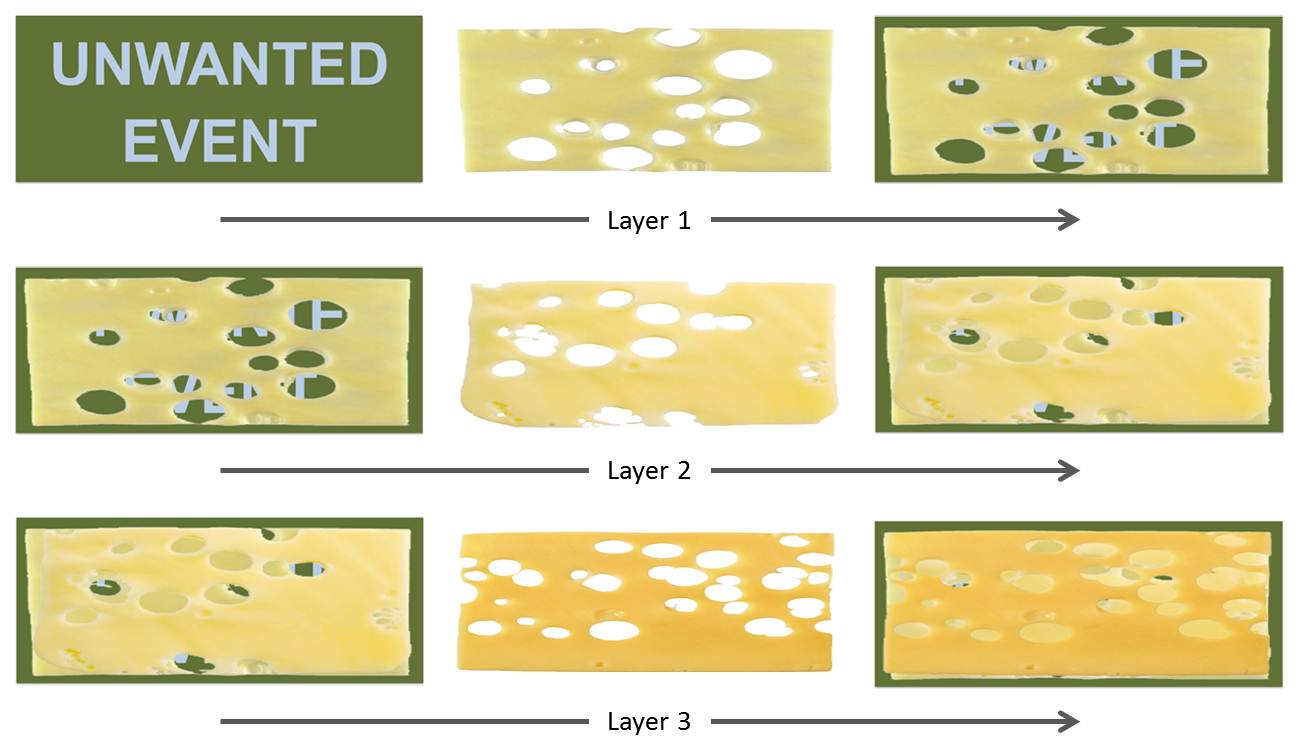
Swiss cheese model: Redundant barriers intended to minimize risk of human errors as defined by Dante Orlandella and James T. Reason of the University of Manchester. It is widely used in aviation, engineering, healthcare, computer security and defence.
The control measures (barriers) that are put in place intended to minimize the risk do not provide complete prevention, this is why redundant controls are necessary to minimize the risk. Like slices of Swiss cheese, the control measures also have holes in them. The system produces failures when a hole in each slice momentarily aligns, permitting (in JT Reason's words) “a trajectory of accident opportunity”, so that a hazard passes through holes in all of the slices, leading to a failure.
The holes in the defences arise for two reasons: active failures and latent conditions. Nearly all adverse events involve a combination of these two sets of factors (JT Reason):
Active failures are the unsafe acts committed by people who are in direct contact with the patient or system. They take a variety of forms: slips, lapses, fumbles, mistakes, and procedural violations. Active failures have a direct and usually short-lived impact on the integrity of the defences. At Chernobyl, for example, the operators wrongly violated plant procedures and switched off successive safety systems, thus creating the immediate trigger for the catastrophic explosion in the core. Followers of the person approach often look no further for the causes of an adverse event once they have identified these proximal unsafe acts. But, as discussed below, virtually all such acts have a causal history that extends back in time and up through the levels of the system.
Latent conditions are the inevitable “resident pathogens” within the system. They arise from decisions made by designers, builders, procedure writers, and top level management. Such decisions may be mistaken, but they need not be. All such strategic decisions have the potential for introducing pathogens into the system. Latent conditions have two kinds of adverse effect: they can translate into error provoking conditions within the local workplace (for example, time pressure, understaffing, inadequate equipment, fatigue, and inexperience) and they can create long-lasting holes or weaknesses in the defences (untrustworthy alarms and indicators, unworkable procedures, design and construction deficiencies, etc.). Latent conditions—as the term suggests—may lie dormant within the system for many years before they combine with active failures and local triggers to create an accident opportunity. Unlike active failures, whose specific forms are often hard to foresee, latent conditions can be identified and remedied before an adverse event occurs. Understanding this leads to proactive rather than reactive risk management.
To use another analogy:active failures are like mosquitoes. They can be swatted one by one, but they still keep coming. The best remedies are to create more effective defences and to drain the swamps in which they breed. The swamps, in this case, are the ever present latent conditions. (JT Reason)
Systematic sampling: A type of random sampling where study units are arranged in some kind of sequence as in a directory or in a series of index cards and a predetermined fraction of the population is selected as the study sample. (WHO)

The users of this electronic publication are free to share (to copy, distribute, display and perform the work and make derivative works based on it only for noncommercial purposes); and to remix (to adapt the work) under the following conditions:
Attribution - The work must be attributed in the manner specified by the author or licensor (but not in a way that suggests that they endorse you or your use of work)