C
Cmax: A term used in pharmacokinetics refers to the maximum (or peak) serum concentration that a drug achieves in a specified compartment or test area of the body after the drug has been administrated and prior to the administration of a second dose.
Calibration: The demonstration that a particular instrument or device produces results within specified limits by comparison with those produced by a reference or traceable standard over an appropriate range of measurements. (ICH Q7)
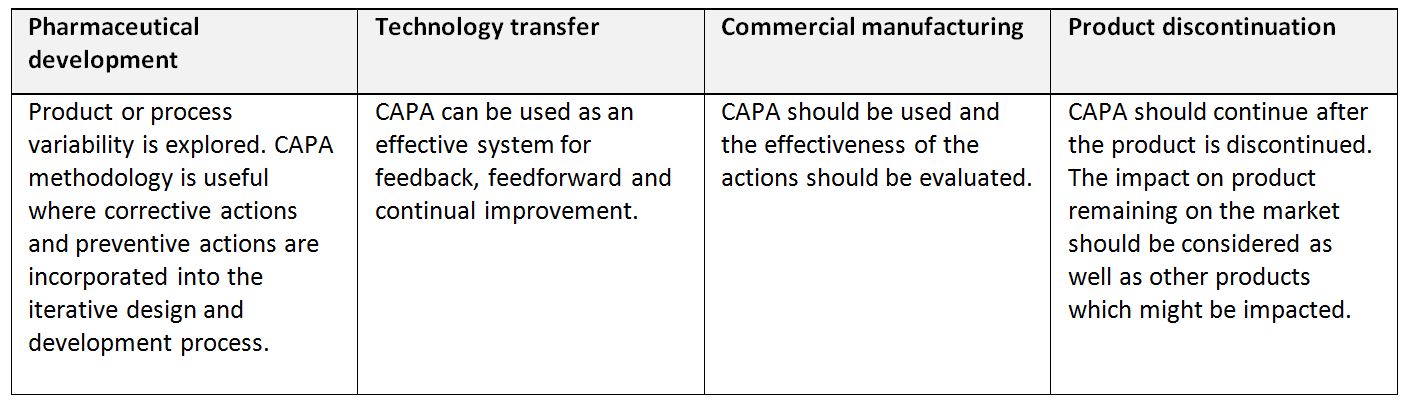
CAPA (Corrective and preventive actions) system: A system for implementing corrective actions and preventive actions resulting from the investigation of complaints, product rejections, non-conformances, recalls, deviations, audits, regulatory inspections and findings, and trends from process performance and product quality monitoring (ICH Q10). A structured approach to the investigation process should be used with the objective of determining the root cause. The level of effort, formality, and documentation of the investigation should be commensurate with the level of risk, in line with ICH Q9 Quality Risk Management. CAPA methodology should result in product and process improvements and enhanced product and process understanding.
Application of corrective action and preventive action system throughout the product lifecycle (ICH Q10)
Care delivery problems: Problems that are due to the direct provision of care. They arise in the process of care, usually actions or omissions by members of staff. Care delivery problems have two essential features: care deviated beyond safe limits of practice; and the deviation had at least a potential direct or indirect eventual adverse outcome for the patient, member of staff or general public. These problems are also called “active failures”. (CA Vincent)
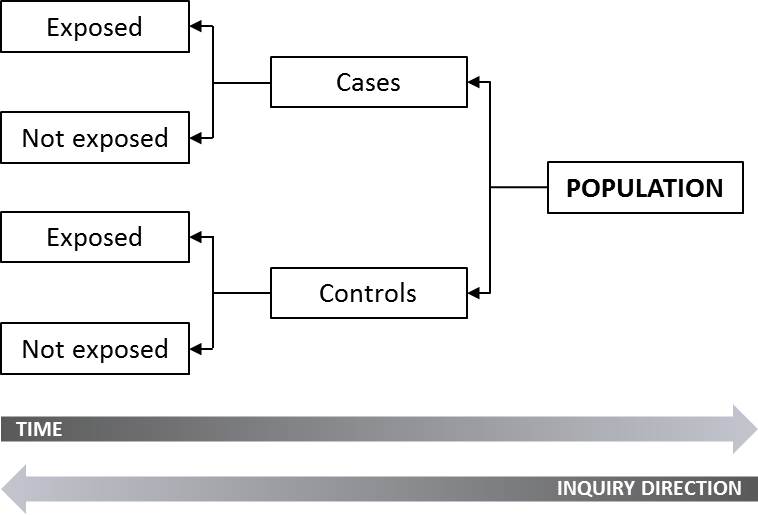
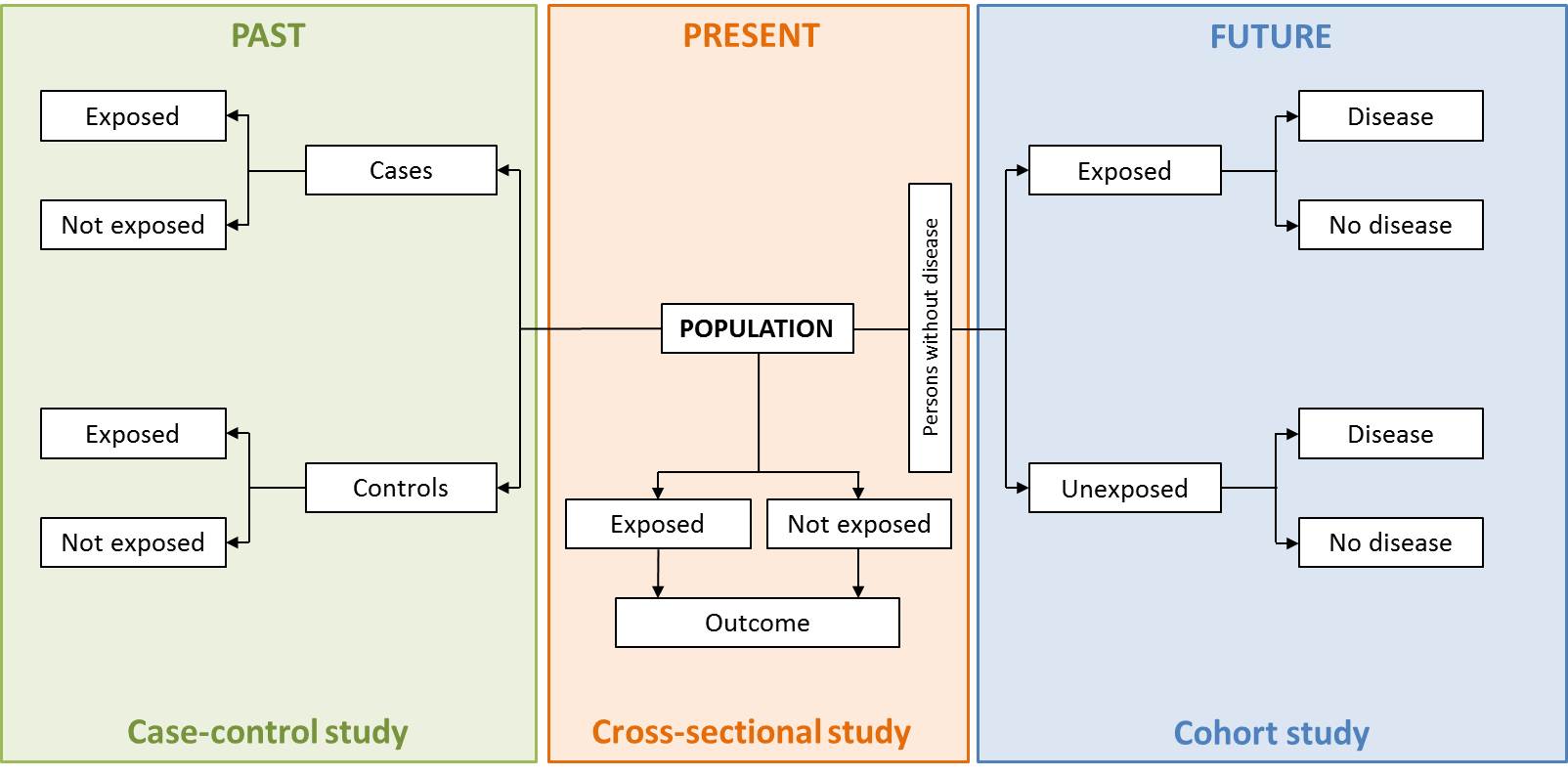
Case-control study: An observational study in which the exposure to a particular risk factor is determined retrospectively, and the effect of this exposure is compared between individuals (the cases) who experience an event and individuals who do not (the controls).
Case definition: A set of diagnostic criteria that must be fulfilled to confirm a case of a particular disease. Case definitions can be based on clinical criteria, laboratory criteria or combinations of the two. (WHO)
Case report form (CRF): A document that is used to record data on each trial subject during the course of the trial, as defined by the protocol. The data should be collected by procedures which guarantee preservation, retention and retrieval of information and allow easy access for verification, audit and inspection. (WHO)
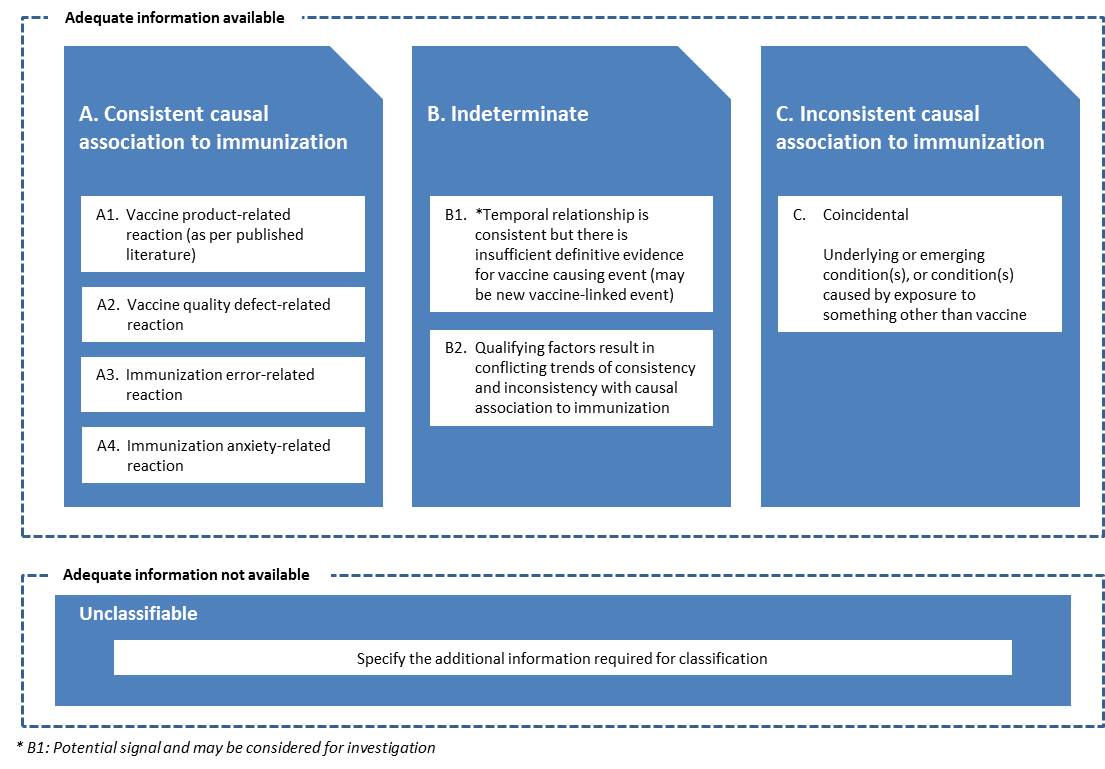
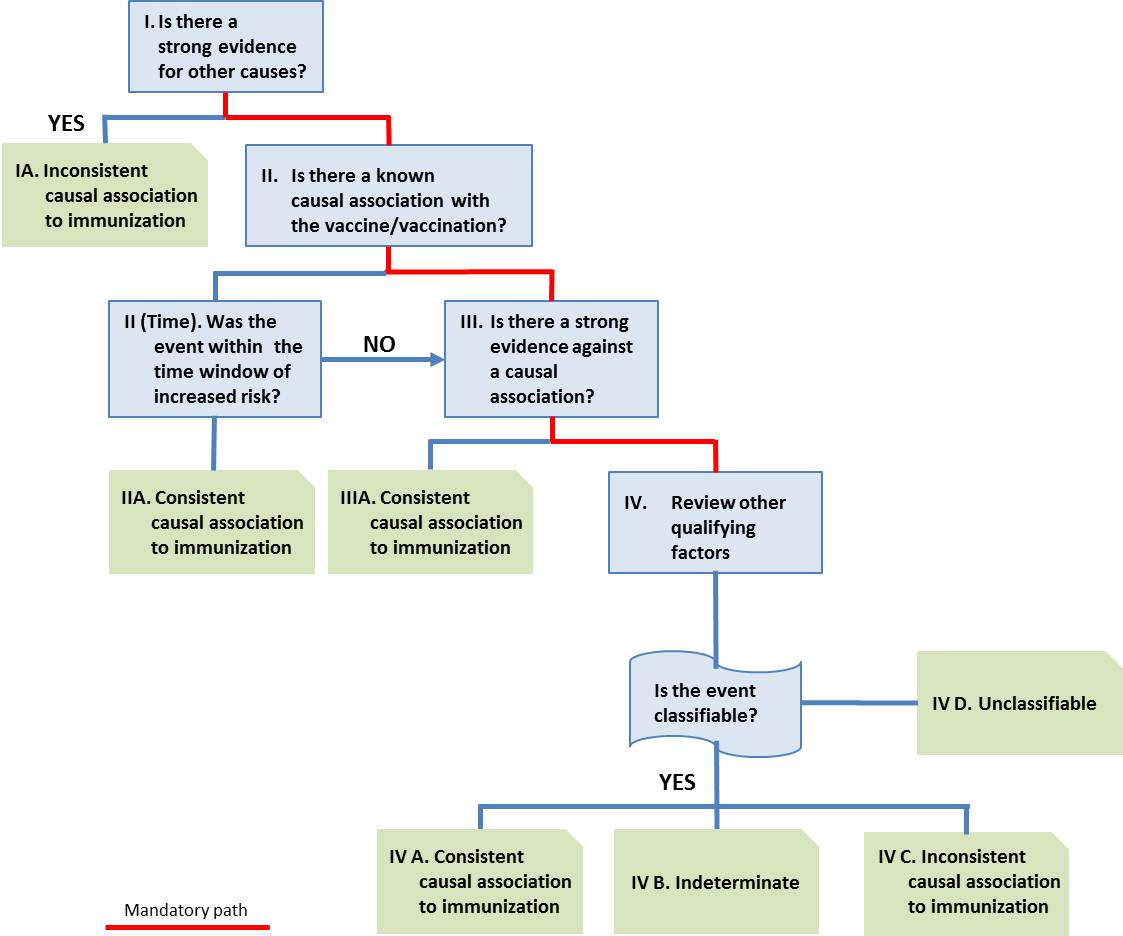
Causal association: A cause-and-effect relationship between a causative factor and a disease with no other factors intervening in the process. (WHO)
Causal factor: A factor that affects an event’s outcome. Causal factor is not a root-cause. See root-cause.
Causality assessment: The systematic review of data about an AEFI case; aiming to determine the likelihood of a causal association between the event and the vaccine(s) received.
The quality of the causality assessment depends upon:
- the performance of the AEFI reporting system in terms of responsiveness, effectiveness and quality of investigation and reports;
- the availability of adequate medical and laboratory services and access to background information
- the quality of the causality review process.
Causality assessment usually will not prove or disprove an association between an event and the immunization. It is meant to assist in determining the level of certainty of such an association. A definite causal association or absence of association often cannot be established for an individual event. (WHO)
Cause: An antecedent set of actions, circumstances or conditions that produce an event, effect, or phenomenon. A cause may be proximate (immediately precede) or remote (a factor in predisposing to) the event, effect, or phenomenon. (Canadian Patient Safety Dictionary)
Cause and effect diagrams: See fish bone diagram and fault-tree analysis.
Center of Excellence for Independent Validators in Pharmaceutical Logistics (CEIV): An IATA project to help organizations and the entire air cargo supply chain to get on the right track to achieve pharmaceutical handling excellence. CEIV Pharma addresses industry’s need for more safety, security, compliance and efficiency to create a globally consistent and recognized pharmaceutical product handling certification. By establishing a common baseline from existing regulations and standards, this certification ensures international and national compliance to safeguard product integrity while addressing specific air cargo needs.
CEIV Pharma encompasses, or even supersedes, many of the existing pharmaceutical standards and guidelines such as:
- IATA Temperature Control Regulations (TCR)
- European Union Good Distribution Practices (EU GDP)
- World Health Organization (Annex 5 of TRS 957)
- United States Pharmacopeia Standards
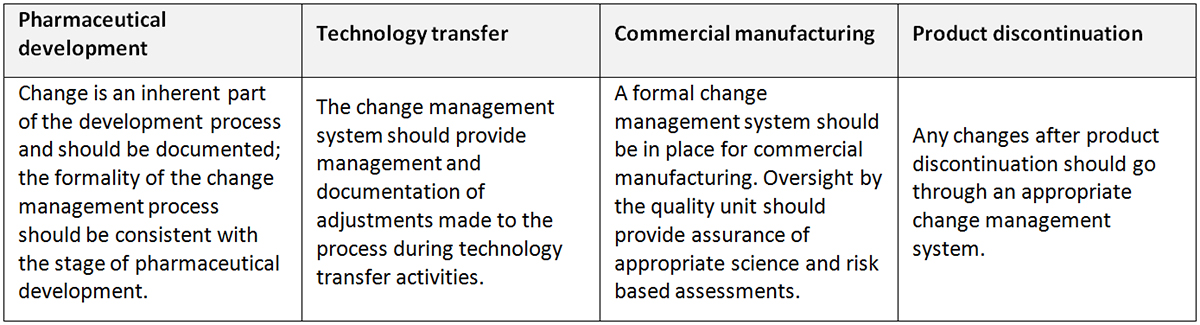
Change management: A systematic approach to proposing, evaluating, approving, implementing and reviewing changes. (ICH Q10)
Change management system: A systematic approach to proposing, evaluating, approving, implementing and reviewing changes. Innovation, continual improvement, the outputs of process performance and product quality monitoring and CAPA drive change. In order to evaluate, approve and implement these changes properly, a company should have an effective change management system. As defined by the ICH Q10, the change management system should include the following, as appropriate for the stage of the lifecycle:
- Quality risk management should be utilised to evaluate proposed changes. The level of effort and formality of the evaluation should be commensurate with the level of risk;
- Proposed changes should be evaluated relative to the marketing authorisation, including design space, where established, and/or current product and process understanding. There should be an assessment to determine whether a change to the regulatory filing is required under regional requirements. As stated in ICH Q8 Pharmaceutical Development, working within the design space is not considered a change (from a regulatory filing perspective). However, from a pharmaceutical quality system standpoint, all changes should be evaluated by a company’s change management system;
- Proposed changes should be evaluated by expert teams contributing the appropriate expertise and knowledge from relevant areas (e.g., pharmaceutical development, manufacturing, quality, regulatory affairs and medical), to ensure the change is technically justified. Prospective evaluation criteria for a proposed change should be set;
- After implementation, an evaluation of the change should be undertaken to confirm the change objectives were achieved and that there was no deleterious impact on product quality.
Application of change management system throughout the product lifecycle (ICH Q10)
Charter: A document prepared by the sponsor, which establishes the role and responsibilities of the DSMB vis-à-vis the sponsor and other parties engaged in the study. (WHO)
Chemical indicators: (also called markers or phase-change indicators), are generally impregnated onto a paperboard substrate. These indicators, sometimes referred to as critical temperature indicators, are based on a phase change or chemical reaction that occurs as a function of temperature. Examples include liquid crystals, waxes, polymers, and lacquers that change phase, and thereby their appearance, as a function of temperature. Chemical temperature threshold indicators are irreversible and are suitable for high or low temperatures. Temperature threshold indicators show a response and typically are single-use devices. These indicators provide a signal only when exposed to temperatures higher than (ascending indicator) or lower than (descending indicator) a predetermined threshold temperature. (WHO)
Examples of chemical indicators (Temptime Corp.)

Chlorofluorocarbons (CFCs): A CFC is an organic compound that contains only carbon, chlorine, and fluorine, produced as a substituted derivative of methane and ethane. It is an ozone-depleting compound, which is highly damaging to the environment. It is now illegal to operate refrigerated vehicles using CFCs as the refrigerating fluid or to have CFCs within the insulation in non-Article 5 countries. WHO recommends that fixed refrigeration equipment and refrigerated vehicles containing CFC’s should not be purchased or operated. (WHO)
Class A packaging: Prior to - and at the time of packing - the vaccines must be kept within the storage temperature limits recommended by the manufacturer. The vaccine must be packed to ensure that the warmest temperature inside the insulated package does not rise above +8°C in continuous outside ambient temperatures of +43°C for a period of at least 48 hours (WHO). See also class ABC packaging.
Class ABC packaging: On the basis of their thermostability and presentation, WHO classifies the vaccines into three categories (A, B and C) for packaging of international shipments. WHO specifies the minimum and maximum acceptable temperatures to which vaccines in each category can be exposed during international transport, for a period of at least 48 hours. See also class A packaging, class B packaging and class C packaging.
WHO classification and temperature criteria for international shipment of vaccines
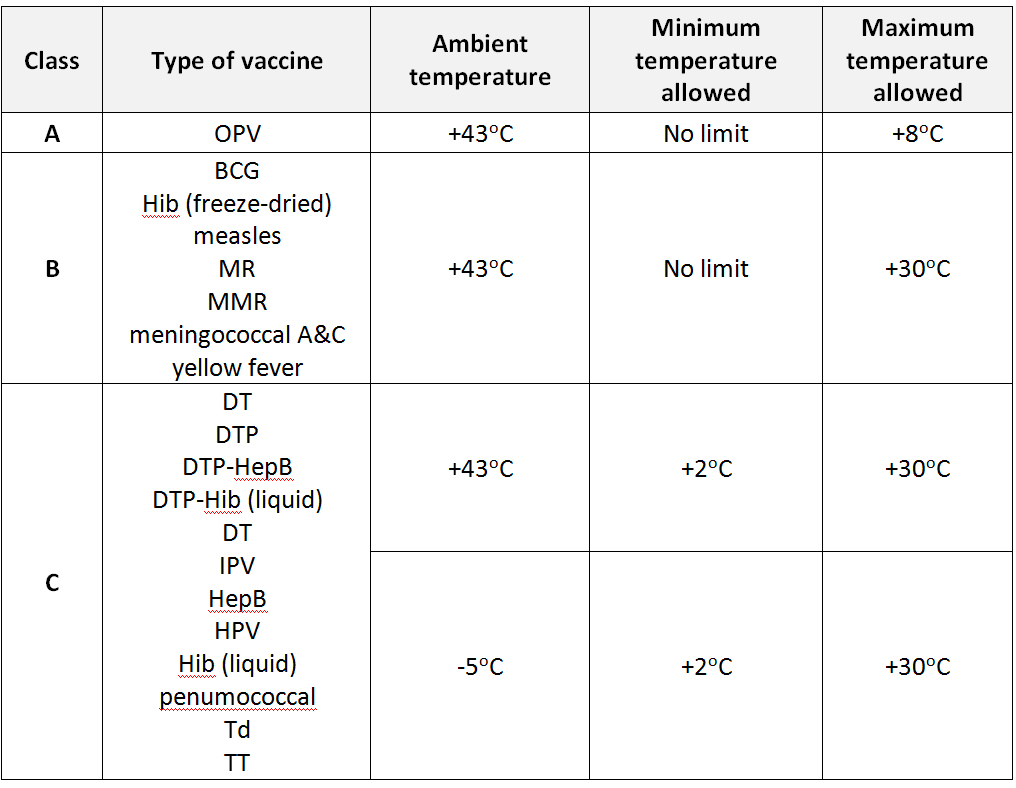
Class B packaging: Prior to - and at the time of packing - the vaccines must be kept within the storage temperature limits recommended by the manufacturer. The vaccines must be packed to ensure that the warmest temperature inside the insulated package does not rise above +30°C in continuous outside ambient temperatures of +43°C for a period of at least 48 hours. Diluents for freeze-dried vaccines must always be included with the vaccine shipment in a quantity that matches the quantity of vaccine; diluents, however, do not require temperature-controlled packaging (WHO). See also class ABC packaging.
Class C packaging: Prior to - and at the time of packing - the vaccines must be kept within the storage temperature limits recommended by the manufacturer. The vaccines must be packed to ensure that the warmest temperature inside the insulated package does not rise above +30°C in continuous outside ambient temperatures of +43°C for a period of at least 48 hours; and the coolest storage temperature of the vaccine does not fall below +2°C in continuous external temperatures of -5°C for a period of at least 48 hours (WHO). See also class ABC packaging.
Client: The organization or individual that is responsible for procuring a building development; sometimes referred to as the employer.(WHO)
Climate zone (for refrigerators): The highest constant ambient temperature at which a WHO prequalified vaccine refrigerator can maintain the vaccine storage compartment between +2oC and +8oC. Established during laboratory testing. (WHO)
The three PQS climate zones are:
- Temperate (up to +27oC)
- Moderate (up to +32C)
- Hot (up to +43oC)
Also see temperature zone symbols for refrigerators.
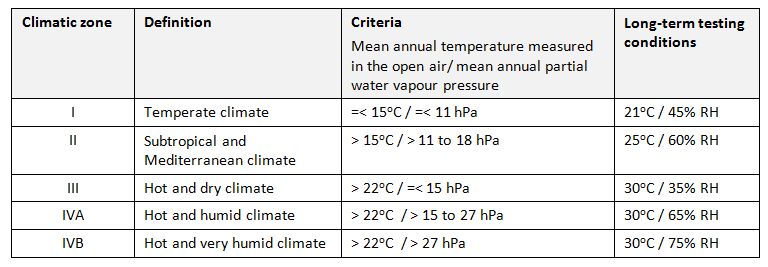
Climatic zone: The zones into which the world is divided based on the prevailing annual climatic conditions. In order to be able to reduce the amount of stability testing required, the number of different long-term testing conditions must be reduced to a sufficient extent (WHO). Four different long-term testing conditions are defined, which match with the climatic conditions of the target markets categorized in just four different climatic zones. This concept is described in regulatory guidelines and pharmacopoeias and has become an established standard in developing finished pharmaceutical products (FPPs).
At the 40th meeting of the WHO Expert Committee on Specifications for Pharmaceutical Preparations held in Geneva in October 2005 (4), it was recommended to split the current Climatic Zone IV (hot and humid) into two zones: Climatic Zone IVA – for which 30°C/65% RH will remain the standard long-term testing condition – and Climatic Zone IVB for which, if justified, 30°C/75% RH will become the long-term testing condition.
Proposed criteria and long-term testing conditions (WHO)
Additional testing conditions i.e., accelerated and – if applicable – intermediate conditions have to be used as described in these guidelines. Selection of the conditions for stability testing is based on a risk analysis. Testing at a more severe long-term condition can be an alternative to storage testing at 25°C/60% RH or 30°C/65% RH. The evaluation of the climatic conditions by each WHO Member State resulted in the recommended storage condition for long-term stability studies shown in the below Table (in some of the countries listed, more extreme conditions are also accepted). The list is grouped by WHO regional offices.
Stability conditions for WHO Member States by Region
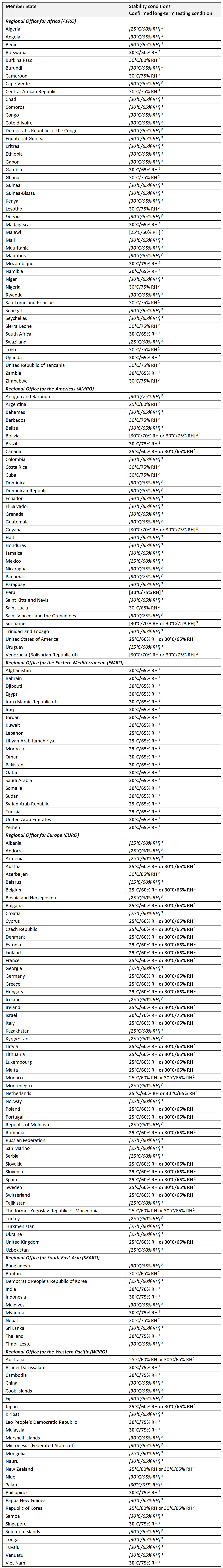
1 Information obtained through respective regional harmonization groups (e.g., ASEAN, ICH and GCC) and from official communications from national medicines regulatory authorities to WHO [entries in bold type].
2 Information collated during the 13th International Conference of Drug Regulatory Authorities (ICDRA), 16–18 September 2008, held in Berne Switzerland, from representatives of national medicines regulatory authorities [entries in normal type].
3 Information provided by the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) [entries in italic type].
Clinical trial/study: Any investigation in human subjects intended to discover or verify the clinical, pharmacological and/or other pharmacodynamic effects of an investigational product(s), and/or to identify any adverse reactions to an investigational product(s), and/or to study absorption, distribution, metabolism, and excretion of an investigational product(s) with the object of ascertaining its safety and/or efficacy. The terms clinical trial and clinical study are synonymous.(ICH E6/R1)
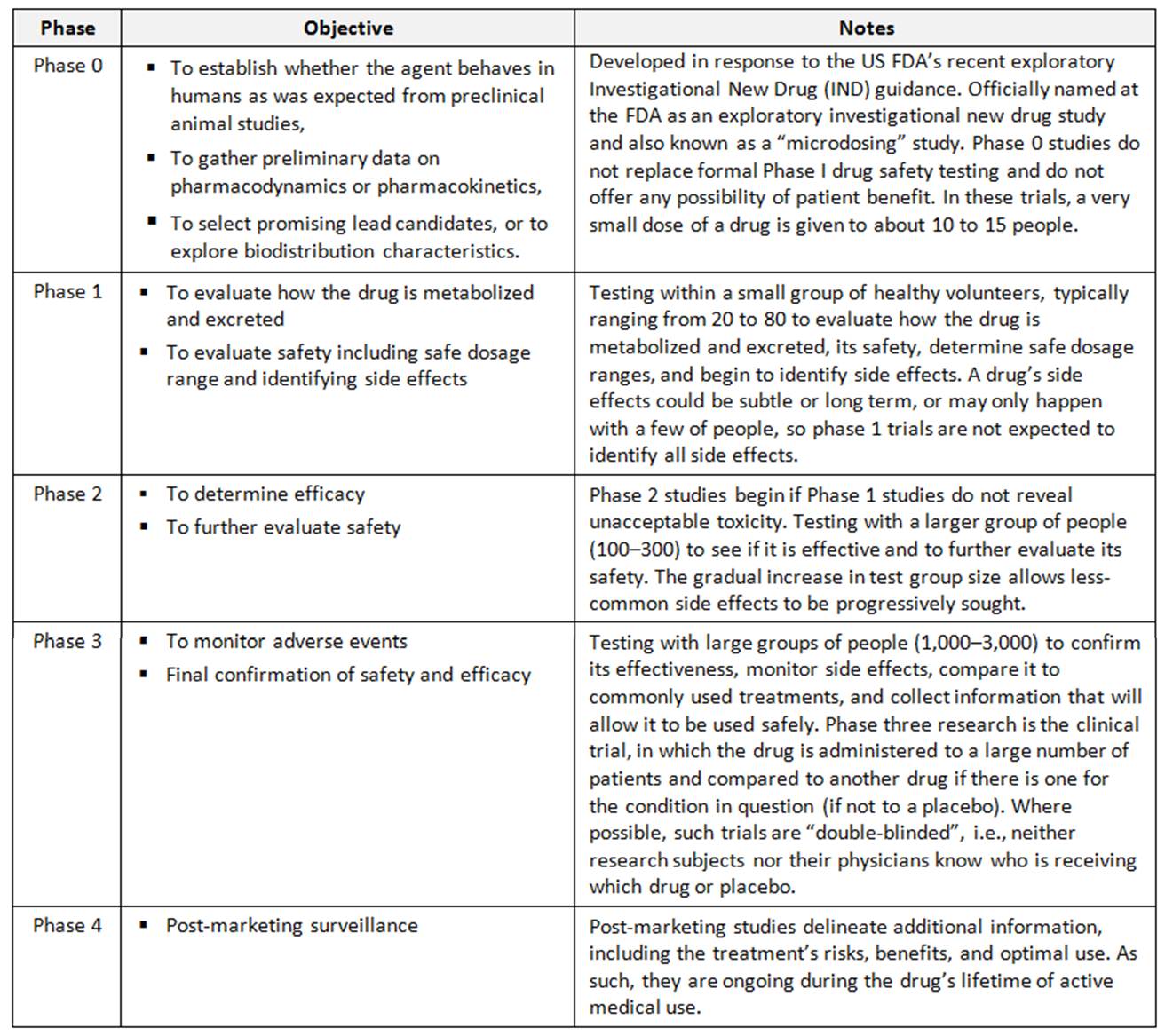
Clinical trials involving new medicinal products are commonly classified into four phases. Each phase is treated as a separate clinical trial. When the investigational product successfully passes through phases 0, 1, 2 and 3, it is approved by the national regulatory authority for use.
Phases of clinical trials
Clinical trials registry: An official platform and catalogue for registering a clinical trial. Some countries require clinical trials being conducted in that country to be registered, others do not. The goal of clinical trials registry is to provide transparency and access to clinical trials, made available to the public.
Cluster: Two or more cases of the same event or similar events related in time, geography, and/or the vaccine administered. (WHO)
Cluster sampling: A sampling methodology that involves: dividing the population into subgroups or clusters that are not necessarily (and preferably not) homogeneous; drawing a random sample of the clusters; and selecting all or a random sample of the persons in each cluster. When each cluster comprises persons in a localized geographic area, such as a county, cluster sampling is especially useful for national surveys. (WHO)
Coefficient of heat transfer: (The “U” value, also referred to as the “K” coefficient in the ATP Agreement) The overall heat transfer of the equipment, defined as the heating power or cooling capacity, W, per degree temperature difference, T, between the internal and external surfaces over the surface of the body, S. (WHO)
The units are W/(m2K) and its formula is below.
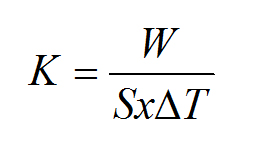
Cohort study: A form of longitudinal study used in various fields including medicine. In medicine, it is an analysis of risk factors and follows a group of people who do not have the disease, and uses correlations to determine the absolute risk of subject contraction. Cohort studies are largely about the life histories of segments of populations, and the individual people who constitute these segments.
Coincidental event: An AEFI that is caused by something other than the vaccine product, immunization error or immunization anxiety. (WHO)
Cold: The condition or subjective perception of having low temperature. Though some define cold as the “absence of heat” it is not fully correct since temperature relates to the thermal energy held by an object, which is the kinetic energy of the random motion of particle constituents of the object, an object will have less thermal energy when it is colder. Absence of heat is only possible at the “absolute zero” point at -273.15oC (zero kelvin) where all motion of the particles are ceased and completely at a resting state.
Cold chain: The entire chain of storage facilities and transportation links through which supplies move from manufacturer to consumer, including port facilities, the primary store, intermediate stores, all service delivery points, equipment and transport vehicles. (WHO)
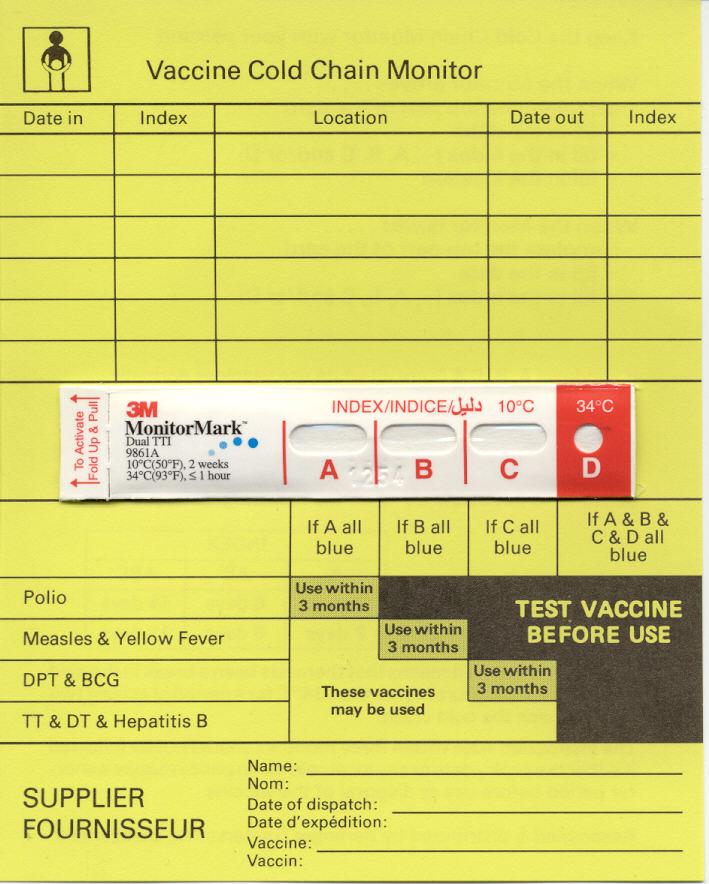
Cold chain monitor (CCM): WHO no longer recommends the use of these cards for in-country use. Their use should now be confined to international shipments only, where dry ice is used; otherwise electronic shipping indicators are generally preferred for this purpose. (WHO)
Cold life: Cold life is measured from the moment when the container lid is closed until the temperature of the warmest point in the vaccine storage compartment first reaches +10°C, at a constant ambient temperature of +43°C. Cold life applies when fully frozen water-packs are used as the coolant; these will continue to be used for transporting OPV and single antigen freeze-dried (lyophilized) vaccines. (WHO)
Cold room: A purpose made insulated enclosure fitted with refrigeration equipment which maintains a set temperature above 0°C. (WHO)
Cold store: A facility where the cold room/freezer room or other refrigeration equipment are located, including a packaging area. (WHO)
Combined vaccine: A vaccine that consists of two or more antigens, combined by the manufacturer at the final formulation stage or mixed immediately before administration. Such vaccines are intended to protect against either more than one disease, or against one disease caused by different strains or serotypes of the same organism. (WHO)
Diphtheria-Tetanus-Pertussis (whole cell)-Hepatitis B-Haemophilus influenzae type b combined vaccine from Serum Institute of India Ltd. (10 dose vial liquid DTwP-HepB + 10 dose vial lyophilised Hib)
Commitment batches: Production batches of an API or FPP for which the stability studies are initiated or completed post-approval through a commitment made in a regulatory application. (WHO)
Commodities: Used interchangeably with stock, goods, products, supplies, and other terms in this manual to refer to all the items that flow through a supply system. (WHO)
Common carrier: A seller of distribution services.
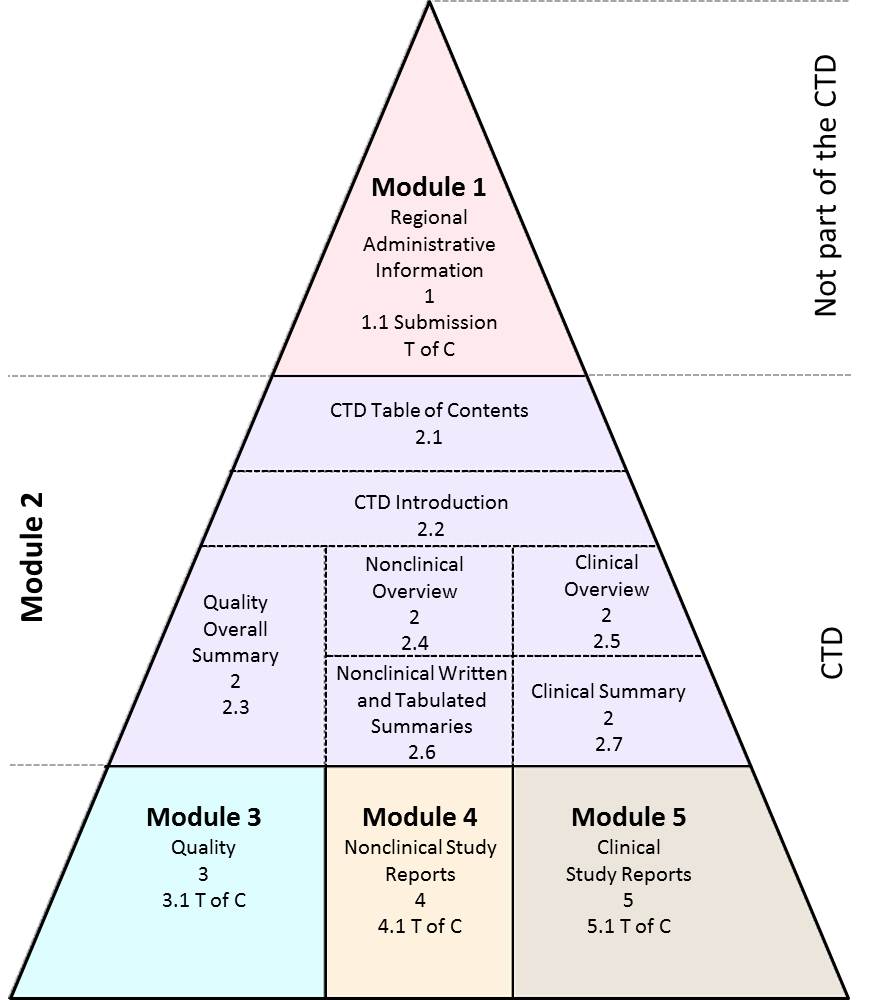
Common technical document (CTD): A set of ICH specification for application dossier for the registration of medicines. The CTD is organized into five modules; Module 1 is region specific and Modules 2-5 are intended to be common for all regions.
Diagrammatic representation of the organization of the ICH Common Technical Document
Community: A community is a group of people understood as having a certain identity due to the sharing of common interests or to a shared proximity. A community may be identified as a group of people living in the same village, town, or country and, thus, sharing geographical proximity. A community may be otherwise identified as a group of people sharing a common set of values, a common set of interests, or a common disease. (WHO)
Community investigation: A population-based trial in large predefined segments of the population to investigate the impact of a treatment on a preventable infectious disease. (WHO)
Commutability: In general terms the concept of commutability seeks to establish the extent to which the reference standard is suitable to serve as a standard for the variety of samples being assayed. The way in which this is done may vary according to the intended application. (WHO)
Comparator product: An investigational or marketed product (i.e., active control), or placebo, used as a reference in a clinical trial (ICH E6/R1). The comparator product is a pharmaceutical product with which the multisource product is intended to be interchangeable in clinical practice. The comparator product will normally be the innovator product for which efficacy, safety and quality have been established. The selection of the comparator product is usually made at the national level by the medicines regulatory authority. See elsewhere for guidance on how to deal with the situation where the comparator proves, on testing, to be of poor quality, e.g., it has poor bioavailability.
Complaint: An expression of dissatisfaction with a product or service and is filed by a customer and received by an organization.
Component: Any major piece, part or assembly of the main equipment or sub-equipment that does not have its own power supply and could not operate as a stand-alone unit (e.g., valves and switches). (WHO)
Compression cycle refrigerator: Refrigerator that uses an electrically powered compressor to drive the cooling system. The electrical supply may come from the grid, a generator or from a renewable source such as solar energy, with or without a battery pack. Provides more reliable and energy-efficient cooling than an absorption cycle refrigerator. (WHO)
Conditioning: Exposing an object to a desired temperature environment to bring its state or situation to a situation with respect to circumstances. Placing water-packs in a freezer to produce ice-packs is a conditioning process. Conditioning is completed when the object reaches thermal equilibrium with the environment it is exposed to. WHO uses the term “conditioning” for frozen icepacks being exposed to room temperature until there is some liquid water in the container, meaning that the ice has reached its phase change point and is at its latent phase.
Conduction: Transfer of heat occurring at molecular level through contact. Some solids, such as metals, are good conductors of heat while others, such as wood, are poor conductors. This is why when one touches a metal and a wooden spoon, metal spoon would feel colder (even though they are at same temperature) because of the high conductivity.
Bakelite handle helps to reduce conduction type heat transfer (Pollapat Chirawong, Shutterstock)

Confidentiality: Prevention of disclosure, to other than authorized individuals, of a sponsor’s proprietary information or of a subject’s identity. (ICH E6/R1)
Conflict of interest: A situation in which a person or organization is involved in financial, emotional or other type interests, one of which could possibly jeopardize his/her (their) ability to provide free and independent advice. Conflict of interest is not declaring a “wrong doing”; it is a situation that many at a time we all fall in. This does not make one act unprofessionally. Many committee members in organizations have to make declaration of their interest. Such declarations are also common for authors in peer-review journals. These declarations are made public so the organization, attendees of meetings as well as readers of peer-review journals are aware of the situation.
Conformity: The fulfilment of a requirement. Requirements may include customer requirements, quality requirements, quality management requirements, management requirements, product requirements, service requirements, contractual requirements, statutory requirements, and regulatory requirements.
Conjugated vaccine: A vaccine produced by covalently binding an antigen to a carrier protein with the intention of improving the immunogenicity of the attached antigen. This technique is most often applied to bacterial polysaccharides for the prevention of invasive bacterial disease.
Pneumococcal 7-valent Conjugate Vaccine - Prevnar: Diphtheria CRM197 Protein manufactured by Pfizer/Wyeth
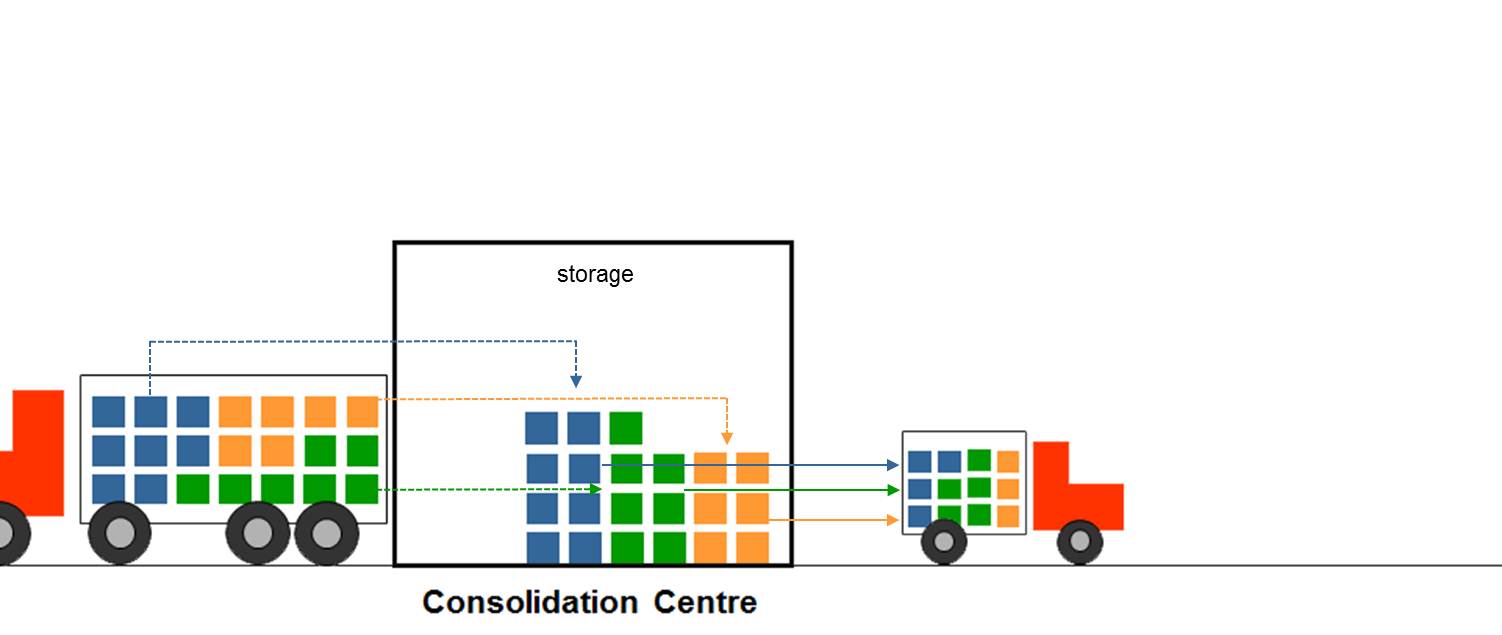
Consolidation centre: A warehouse where multiple products are held so orders can be picked for onward delivery to customers. In a typical immunization supply chain the primary and intermediate storage facilities are consolidation centre type warehouses. (WHO)
Constitution: A constitution sets forth fundamental principles about how a particular entity is constituted and governed. Most governmental systems have constitutions, as do some international organizations (including the WHO) and private associations. Constitutions authorize the entity to engage in particular actions and may also establish specific limits on the entity’s use of its powers.
Governmental constitutions typically include provisions establishing the law-making process, allocating responsibility among governmental actors, and protecting individual liberties and minority rights. Most governmental constitutions are codified - i.e., they are assembled into a single, organized system of written rules. (WHO)
Consumption records: Records kept on products consumed. See also stock keeping records and transaction records.
Contact: An individual who has had contact with an infected person (case) in a way that is considered as having caused significant exposure and therefore a risk of infection.(WHO)
Container closure system: The sum of packaging components that together contains and protects the dosage form. This includes primary packaging components and secondary packaging components, if the latter are intended to provide additional protection to the FPP. A packaging system is equivalent to a container closure system.(WHO)
Contamination: The undesired introduction of impurities of a chemical or microbiological nature, or of foreign matter, into or onto a raw material, intermediate, or API during production, sampling, packaging or repackaging, storage or transport. (ICH Q7A)
Continuous process validation: An alternative approach to process validation in which manufacturing process performance is continuously monitored and evaluated. (ICH Q8/R2)
Contract: A written, dated, and signed agreement between two or more involved parties that sets out any arrangements on delegation and distribution of tasks and obligations and, if appropriate, on financial matters. The protocol may serve as the basis of a contract. (ICH E6/R1)
Contract manufacturer: A manufacturer performing some aspect of manufacturing on behalf of the original manufacturer. (WHO)
Contract research organization (CRO): A person or an organization (commercial, academic, or other) contracted by the sponsor to perform one or more of a sponsor’s trial-related duties and functions. (ICH E6/R1)
Contributing cause: A factor, situation, or agent that accelerates or intensifies the occurrence of the unwanted event. If the contributing cause is removed, it does not prevent the unwanted event from occurring. (J Vesper)
Contributing factors: The reason(s), situational factor(s), or latent condition(s) that played a role in the genesis of an adverse outcome. (Canadian Patient Safety Dictionary)
Control: Any comparator suitable for validation of the trial. The comparator may be either an active treatment or a placebo control. (WHO)
Control (of disease): The reduction of disease incidence, prevalence, morbidity or mortality to a locally acceptable level as a result of deliberate efforts; continued intervention measures are required to maintain the reduction. Example: The Integrated Global Action Plan for the Prevention and Control of Pneumonia and Diarrhoea (GAPPD) by WHO/UNICEF.
Control strategy: A planned set of controls, derived from current product and process understanding that assures process performance and product quality. The controls can include parameters and attributes related to drug substance and drug product materials and components, facility and equipment operating conditions, in-process controls, finished product specifications, and the associated methods and frequency of monitoring and control.(ICH Q10)
Controlled or hazardous products: TTSPPs and other products with high illicit value: poisons, narcotics, psychotropic products, inflammable or explosive substances and radioactive materials. (WHO)
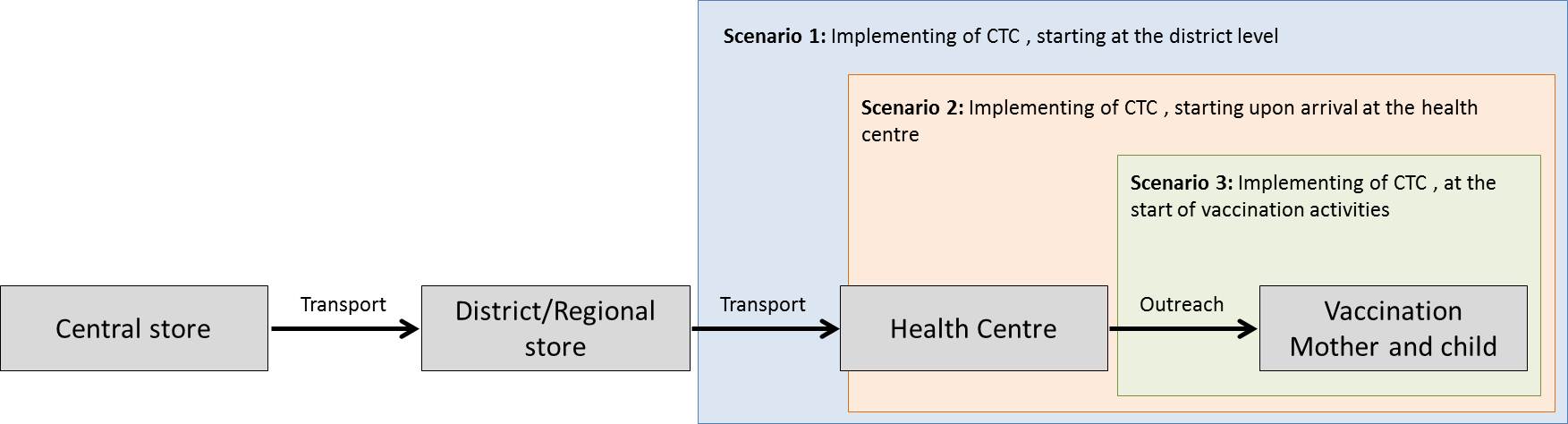
Controlled temperature chain (CTC): An innovative approach to vaccine management allowing vaccines to be kept at temperatures outside of the traditional cold chain of +2°C to +8°C for a limited period of time under monitored and controlled conditions, as appropriate to the stability of the antigen. A CTC typically involves a single excursion of the vaccine into ambient temperatures not exceeding +40°C and for duration of a specific number of days, just prior to administration. (WHO)
The WHO has established the following programmatic criteria for a vaccine to be labelled for and used in a CTC:
- The vaccine should be used in a campaign or special strategy setting. CTC is not currently recommended for immunization through routine delivery.
- The vaccine must be able to tolerate ambient temperatures of at least +40°C for a minimum of three days and should be accompanied by:
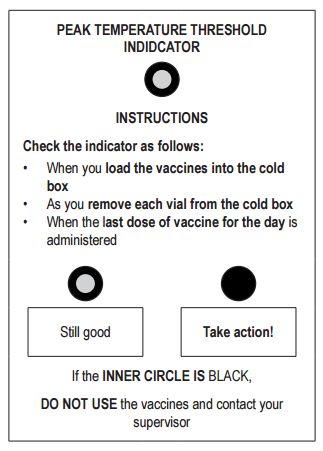
- a. a vaccine vial monitor (VVM) on each vial, and
- b. a peak threshold indicator in each vaccine carrier
- The vaccine must be licensed for use in a CTC by the relevant regulatory authorities, with a label that specifies the conditions.
Possible scenarios for implementation of CTC as defined by WHO (Kartoglu)
Peak threshold temperature indicator used in CTC (Temptime Corp.)
CTC was integrated into Meningococcal A conjugate vaccine mass preventive campaigns for the first time in three West African countries in 2014.
For details on the CTC policy, see http://www.who.int/immunization/programmes_systems/supply_chain/resources/tools/en/index6.html
Controller: A device that interprets a mechanical, digital or analogue signal, by a sensor, to control an equipment or component. (WHO)
Controller, critical: A controller for which control has a direct impact on the quality of the product or proper operation of the equipment. (WHO)
Controller, non-critical: A controller for which control has no direct impact on the quality of the product or proper operation of the equipment. (WHO)
Convection: Transfer of heat from one place to another by the movement of fluids and air. Convection is the circular motion that happens when warmer air or liquid which has faster moving molecules, making it less dense rises, while the cooler air or liquid drops down.
Convection in a pot over gas stove (Roman Sigaev, Shutterstock)

Cool: Fairly low temperature.
Cool down time: The time required for a WHO pre-qualified vaccine refrigerator to cool down to within the acceptable temperature range of +2°C to +8°C, measured from the moment when the appliance is initially turned on. Established by laboratory testing at the ambient temperature of the climate zone for which the appliance is prequalified. (WHO)
Cool life: Cool life with cool water-packs at +5°C: Cool life is measured from the moment when the container is closed, until the temperature of the warmest point inside the vaccine storage compartment first reaches +20°C, at a constant ambient temperature of +43°C. Cool life applies when cool water-packs are used. (WHO)
Cool life test: The empty passive container is stabilized at +43.0°C and loaded with cool water-packs which have been stabilized at + 5.0°C for a minimum of 24 hours. Cool life is measured from the moment when the container is closed, until the temperature of the warmest point inside the storage compartment first reaches +20.0°C, at a constant ambient temperature of +43.0°C. (WHO)
Cool water-pack: A water-pack cooled to a temperature of between +2.0°C and +8°C before use. (WHO)
The only way to eliminate the freezing risk entirely is to transport liquid vaccines, other than OPV, in cold boxes lined with cool water-packs which have been precooled in a refrigerator to a temperature of +2°C to +8°C. Where it is essential to transport OPV, liquid and freeze-dried vaccines in a single carrier, experiments have shown that cool water-packs may safely be used provided the cool life of the carrier is not exceeded. Changing over to the use of cool water-packs involves significant changes in practice. In addition there are equipment implications because additional refrigerators will be needed at primary and sub-national level to cool the water-packs in bulk.
Coolant: Ice, water, water-based gel, phase-change material, dry ice, or other substance, typically encapsulated in a rigid or flexible plastic container, used to maintain a predefined temperature range inside a passive container during transport operations. (WHO)
Cooling: The process of removing heat from a system by exposing it to an environment (or another system) that is at lower temperature. Cooling occurs through the transfer of thermal energy through radiation, convection and conduction.
Coordinating investigator: An investigator assigned the responsibility for the coordination of investigators at different centres participating in a multicentre trial. (ICH E6/R1)
Correction: A correction is any action that is taken to eliminate nonconformity or other undesirable situation. However, corrections do not address root causes. When applied to products, corrections can include reworking products, reprocessing them, regrading them, assigning them to a different use, or simply destroying them.
Corrective action: Action to eliminate the cause of a detected nonconformity or other undesirable situation. Corrective action is taken to prevent recurrence. (ISO 9001:2015)
Cost benefit analysis: A conceptual framework applied to any systematic, quantitative appraisal of a public or private project to determine whether, or to what extent, that project is worthwhile from a social perspective. Cost-benefit analysis differs from a straightforward financial appraisal in that it considers all gains (benefits) and losses (costs) to social agents. Cost benefit analysis usually implies the use of accounting prices. (EU)
Cost effectiveness analysis: An appraisal and monitoring technique used when benefits cannot be reasonably measured in money terms. It is usually carried out by calculating the cost per unit of “non-monetised” benefit and is required to quantify benefits but not to attach a monetary price or economic value to the benefits. (EU)
Court decisions: Courts exist to hear criminal cases and to resolve disputes between private parties and challenges to governmental actions. In civil law countries (such as France), courts are required to decide all cases by reference to the published legal code. In common law countries (such as the United States), courts rely on the legal code if it is applicable, but if no code provision applies they base their decision on “precedents,” or previous decisions in analogous cases decided by other judges. An important part of legal decision-making in common-law countries involves identifying relevant prior cases and determining how their reasoning applies to a current dispute. (WHO)
Critical control point (CCP): A step or procedure at which controls or checks can be applied to prevent or reduce a hazard or risk to an acceptable or critical level. In the context of distribution and handling of time- and temperature-sensitive pharmaceutical products, CCPs are typically defined for those activities where time and temperature abuse may occur or where critical processes that can affect the performance of the packaging solution or containment system are at risk. (WHO)
Critical deviation: A variation to previously established critical parameters or a significant variation to standard operations which could affect the quality of the API or intermediate. Critical deviations should always be investigated and corrective actions identified. (USFDA)
Critical process parameter (CPP): A process parameter whose variability has an impact on a critical quality attribute and therefore should be monitored or controlled to ensure the process produces the desired quality.(ICH Q8)
Critical quality attribute (CQA): A physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality. (ICH Q8)
Cross-contamination: Contamination of a material or product with another material or product.
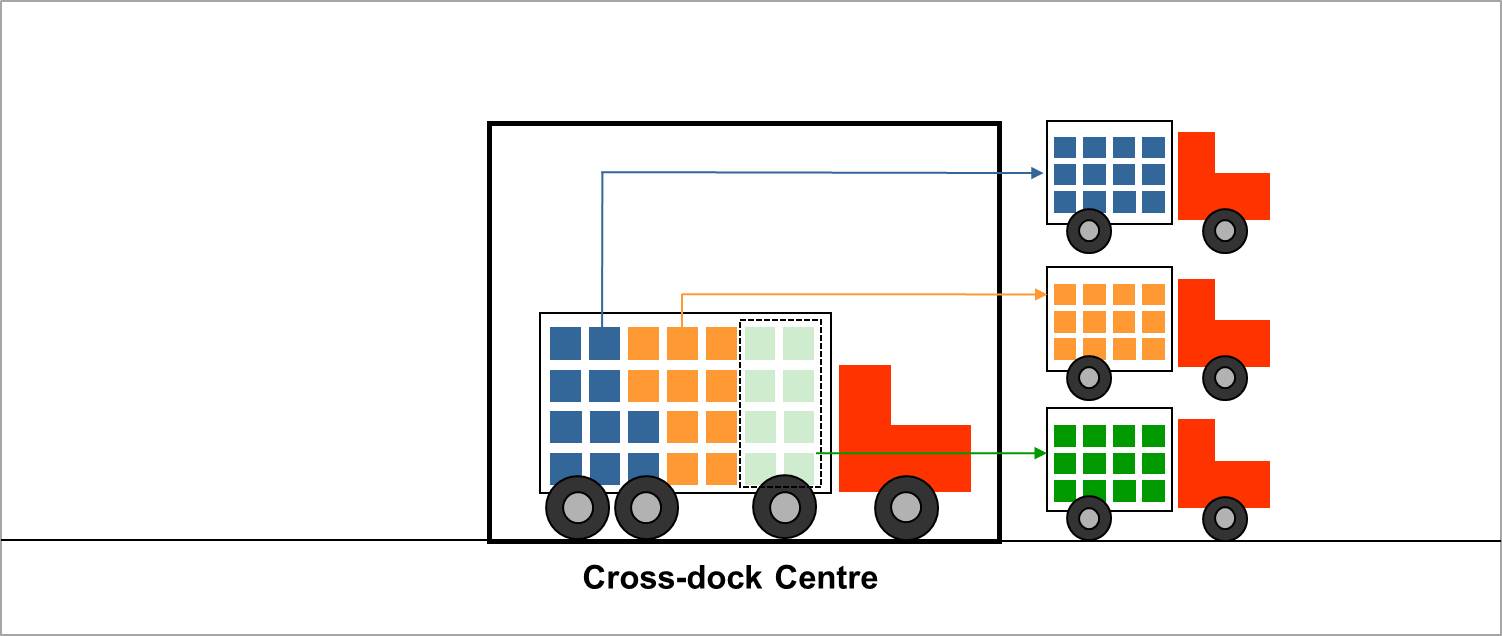
Cross-dock centre: There is no warehousing in cross-dock centres. In cross-dock centres, there is a direct transfer from incoming to outgoing vehicle without any storage taking place at all. (WHO)
Cross-sectional study: A form of observational study carried out just one point in time or over a short period of time. Also known as transversal study or prevalence study. Associations found in cross-sectional studies are difficult to interpret.
Cryogenic dry/vapour shipper: A temperature-controlled insulated packaging container or system compatible with liquefied gases such as nitrogen used for maintaining extremely low temperatures during shipping. A porous medium internal to the shipping container absorbs and contains all the free flowing liquid and does not allow it to come into contact with the product - a process known as “charging”. A fully charged and undamaged dry/vapour shipper containing nitrogen can maintain -196°C for up to 10 days, depending on the unit size. (WHO)
Cycle stock: See working stock

The users of this electronic publication are free to share (to copy, distribute, display and perform the work and make derivative works based on it only for noncommercial purposes); and to remix (to adapt the work) under the following conditions:
Attribution - The work must be attributed in the manner specified by the author or licensor (but not in a way that suggests that they endorse you or your use of work)