B
Bacteria: Single-celled microorganisms which can exist either as independent (free-living) organisms or as parasites (dependent upon another organism for life).
Baseline samples: Samples that are retained under optimal storage conditions to retain biological or immunological activity and that are used for comparison purposes. The baseline samples will need to be stored at a lower temperature than that used for the reference standard. (WHO)
Batch: A defined quantity of starting material, packaging material or finished pharmaceutical product (FPP) processed in a single process or series of processes so that it is expected to be homogeneous. It may sometimes be necessary to divide a batch into a number of sub-batches, which are later brought together to form a final homogeneous batch. In the case of terminal sterilization, the batch size is determined by the capacity of the autoclave. In continuous manufacture, the batch must correspond to a defined fraction of the production, characterized by its intended homogeneity. The batch size can be defined either as a fixed quantity or as the amount produced in a fixed time interval. (ICH Q7)
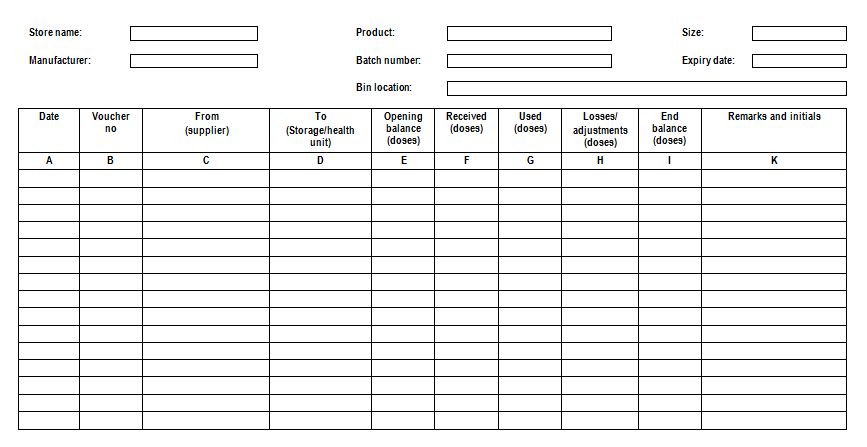
Batch card: A stock keeping record that keeps information about a single lot of a product. Also known as “bin card”.
A sample batch card (WHO)
Batch number (or lot number): A unique combination of numbers, letters, and/or symbols that identifies a batch (or lot) and from which the production and distribution history can be determined. (ICH Q7)
Batch release: See lot release.
Batch release certificate: See lot release certificate.
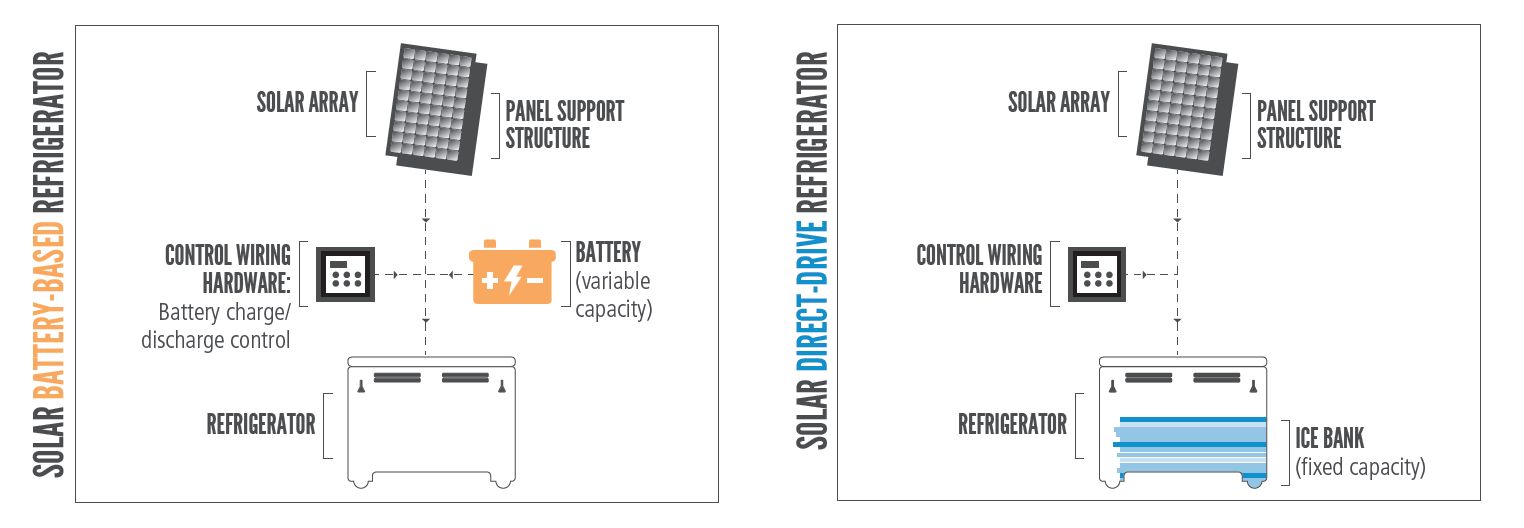
Battery powered solar refrigerator: Refrigerators that use solar energy stored in a battery to drive the cooling system, even during periods when solar irradiance is unavailable or limited (i.e., at night or on cloudy days).(WHO)
Schematic showing difference between battery-powered and solar direct-drive refrigerators (WHO)
Beneficence: Maximizing benefits and minimizing harms and wrongs. (WHO)
Beta error:See type II error and validity.
Bias (statistical & operational): The systematic tendency of any factors associated with the design, conduct, analysis and evaluation of the results of a clinical trial to make the estimate of a treatment effect deviate from its true value. Bias introduced through deviations in conduct is referred to as “operational” bias. The other sources of bias listed above are referred to as “statistical”.(ICH E9)
Bin card: See batch card.
Bioburden: The level and type (e.g., objectionable or not) of micro-organisms that can be present in raw materials, API starting materials, intermediates or APIs. Bioburden should not be considered contamination unless the levels have been exceeded or defined objectionable organisms have been detected. (ICH Q7)
Biodistribution study: A preclinical animal study designed to determine the distribution of a vector to sites other than the intended therapeutic site. (WHO)
Bioequivalence: Two pharmaceutical products are bioequivalent if they are pharmaceutically equivalent or pharmaceutical alternatives, and their bioavailabilities, in terms of peak (Cmax and Tmax) and total exposure (area under the curve) after administration of the same molar dose under the same conditions, are similar to such a degree that their effects can be expected to be essentially the same. (WHO)
Bioequivalence test: A test that determines the equivalence between the multisource product and the comparator product using in vivo and/or in vitro approaches. (WHO)
Biological indicators: The use of organisms to test the efficacy of sterilization processes.
Biological tests (bioassay): A procedure for the estimation of the nature or potency of a material by means of the reaction that follows its application to some elements of a living system (examples include animals, tissues, cells, receptors and enzymes). The potency of the material being measured is often defined in IUs or, in some circumstances, may be defined in SI units, by comparison with the reaction of the system to that of a biological reference preparation. (WHO)
BIPM: Bureau International des Poids et Mesures (International Bureau of Weights and Measures). Created in 1875, an international standards organization, one of three such organizations established to maintain the International System of Units under the terms of the Metre Convention (Convention du Mètre). For details see http://www.bipm.org/
Blind review: The checking and assessment of data during the period o time between trial completion (the last observation on the last subject) and the breaking of the blind, for the purpose of finalizing the planned analysis. (ICH E9)
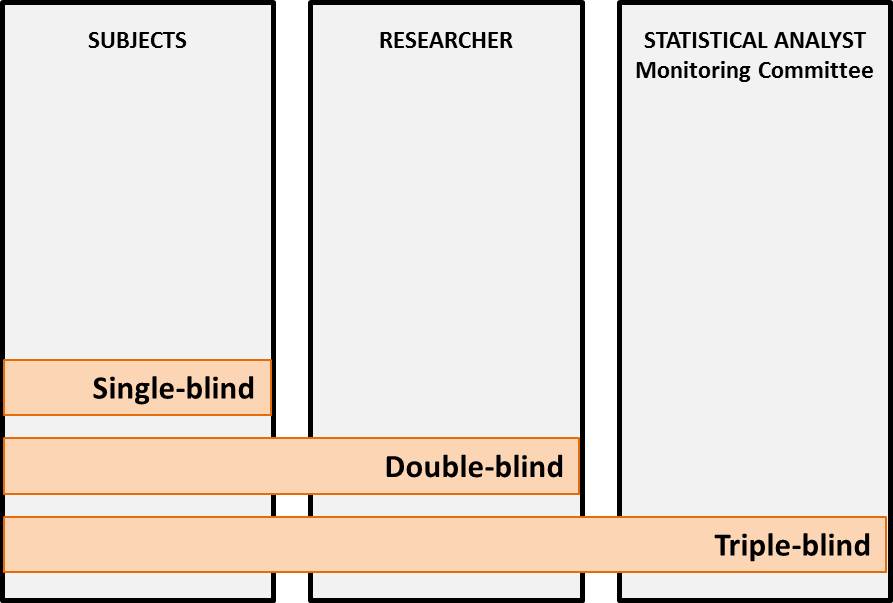
Blinded/unblinded (data): Data (or their format/presentation) are considered “blinded” when those with access to the data are not informed of the significant characteristics associated with them. Often this refers to identification of the intervention associated with the data. Data (or their format/presentation) are considered “unblinded” when those with access to them are informed of the significant characteristics (e.g., intervention) to which the data are associated. Single, double or triple “blinding” is used in experiments to minimize or eliminate the bias.
Blinding: A procedure in which one or more parties to the trial are kept unaware of the treatment assignment(s). Single-blinding usually refers to the subject(s) being unaware of the treatment assigned to them, and double-blinding usually refers to the subject(s), investigator(s) and, in some cases, data analyst(s) being unaware of the treatment assignment. (ICH E6/R1)
Booster vaccination: Vaccination given at a certain time interval after primary vaccination to enhance immune responses and induce long-term protection. (WHO)
Bracketing: The design of stability schedule such that only samples at the extremes of certain design factors, e.g., strength and package size, are tested at all-time points as in a full design. The design assumes that the stability of any intermediate levels is represented by the stability of the extremes tested. Where a range of strengths is to be tested, bracketing is applicable if the strengths are identical or very closely related in composition (e.g., for a tablet range made with different compression weights of a similar basic granulation, or a capsule range made by filling different plug fill weights of the same basic composition into different size capsule shells). Bracketing can be applied to different container sizes or different fills in the same container closure system. (WHO)
Bridging studies: Studies intended to support the extrapolation of efficacy, safety and immunogenicity data from one formulation, population or dose regimen to another. (WHO)
Buffer stock: See safety stock.
Bulk purified plasmid (drug substance): The purified plasmid before final formulation. It is obtained from one or more bulk harvests, and is kept in one or more containers designated as a single homogeneous production lot and used in the preparation of the final dosage form (drug product). (WHO)
Bulking factor: The coefficient used for calculation of volume requirements in insulated packaging, the volume of the package divided by the volume of the product it contains. In vaccine shipments, insulated packaging occupies up to 8.5 times the volume of the vaccine that it contains. WHO issues bulking factors for insulated packaging based on its prequalified vaccines list. For further details see Guidelines on the international packaging and shipping of vaccines (WHO/IVB/05.23) http://apps.who.int/iris/bitstream/10665/69368/1/WHO_IVB_05.23_eng.pdf

The users of this electronic publication are free to share (to copy, distribute, display and perform the work and make derivative works based on it only for noncommercial purposes); and to remix (to adapt the work) under the following conditions:
Attribution - The work must be attributed in the manner specified by the author or licensor (but not in a way that suggests that they endorse you or your use of work)