T
Tmax: The amount of time that a drug is present at the maximum concentration in serum.
Technical agreement: See service level agreement.
Technical Report Series (TRS): A series publication by WHO on reports and recommendations including all adopted guidelines in the form of Annexes from WHO Expert Committees, endorsed by the WHO Executive Board.
ECBS and ECSPP expert committees reports in Technical Report Series (WHO)


Temperature control device: A device which actively controls the operation of cooling plant used to store or transport TTSPPs. (WHO)
Temperature Control Regulations (TCR): A comprehensive guide published by the IATA, designed to enable stakeholders involved in the transport and handling of pharmaceutical product to safely meet the requirements. The TCR provides you access to the most current and efficient practices for your perishable cargo operations and an integral tool to achieve cost savings and avoiding delays by guaranteeing your shipments are problem-free and compliant with international or local regulations. The TCR includes:
- Up-to-date airline and government requirements pertaining to the transport of healthcare and pharmaceutical products
- Requirements on handling, marking & labeling
- Necessary packaging requirements
- Information on handling procedures
- The necessary documentation needed when transporting healthcare products
Temperature-controlled: Includes any environment in which the temperature is actively or passively controlled at a level different from that of the surrounding environment within precise predefined limits. (WHO)
Temperature excursion: An event in which a TTSPP is exposed to temperatures outside the range(s) prescribed for storage and/or transport. Temperature ranges for storage and transport may be the same or different; they are determined by the product manufacturer, based on stability data. In situations in which cool water-packs are used for vaccine transport, an excursion up to a maximum of +20°C is acceptable. (WHO)
Temperature-modified: Includes any environment in which the temperature is predictably maintained at a level different from that of the surrounding environment, but is not actively or passively controlled within precise predefined limits. (WHO)
Temperature monitoring: A process to ensure products remain within recommended temperature range during their storage and transport by using variety of devices and tools. It is essential that the temperature monitoring process is not purely mechanical. Personnel must be made responsible for their actions and must know how to react effectively to problems as soon as they arise. (WHO)
The table below shows the recommended temperature monitoring devices for the fixed storage locations in a typical vaccine supply chain.
Recommended temperature monitoring devices for vaccine supply chain (WHO)
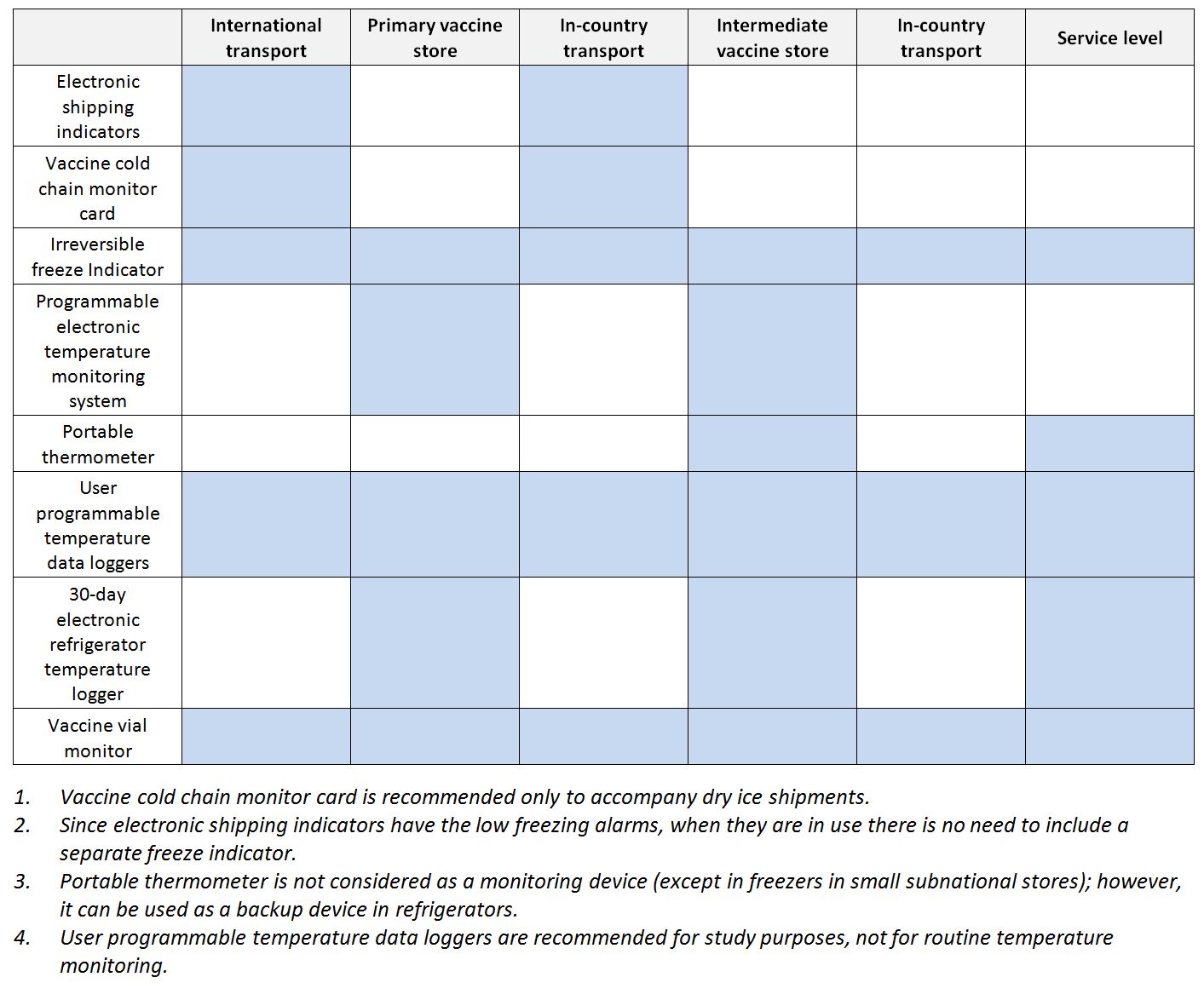
Recommended temperature monitoring devices for the fixed storage locations in a typical vaccine supply chain (modified from WHO)
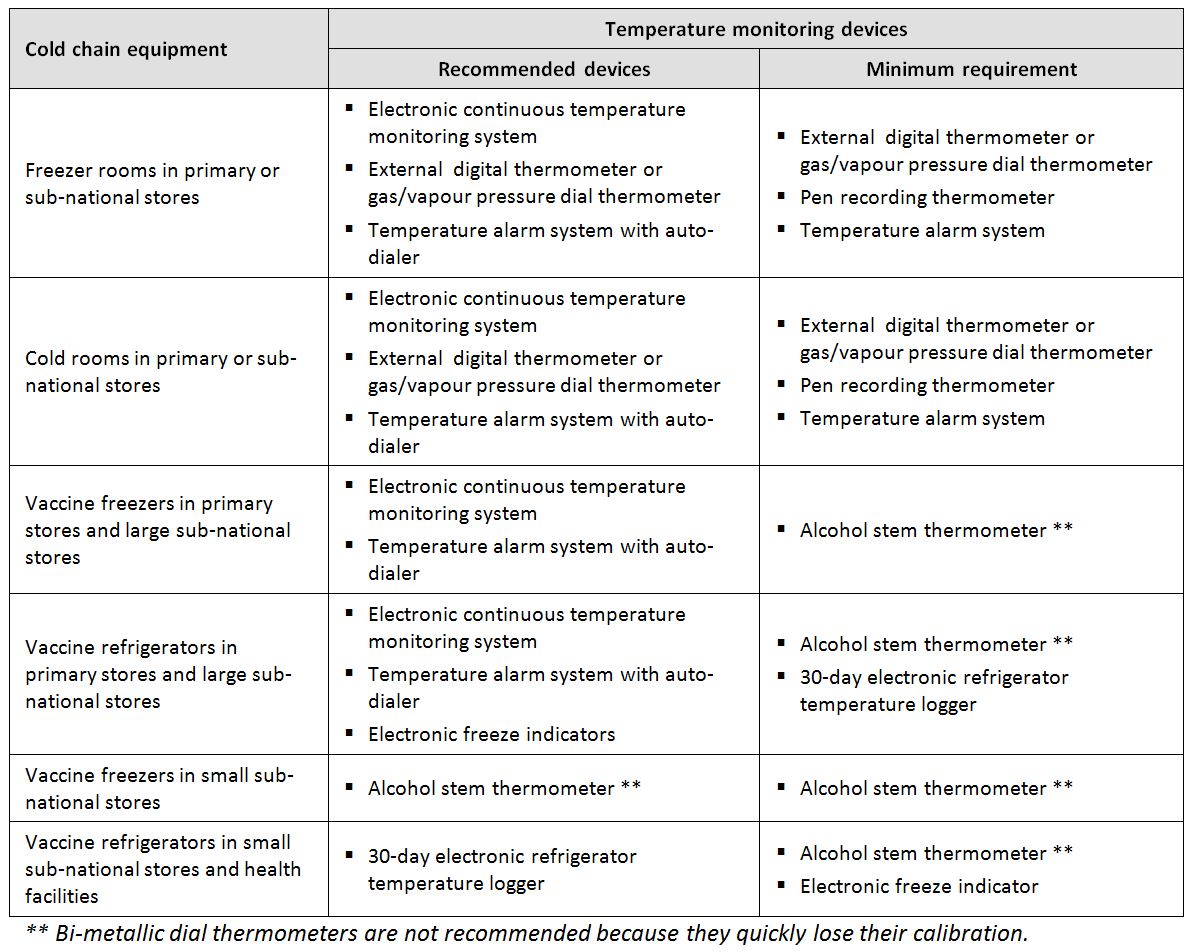
Temperature monitoring device: A device which monitors the temperature of spaces used to store or transport TTSPPs. (WHO)
Temperature stabilizing medium: Ice or gel packs; gel bricks, bottles or pouches; cool water or warm water packs, phase change materials, dry ice, rapid evaporation media which limit exposure of packed product to excessively high or low temperatures during transport: also referred to as refrigerants or coolants. (WHO)
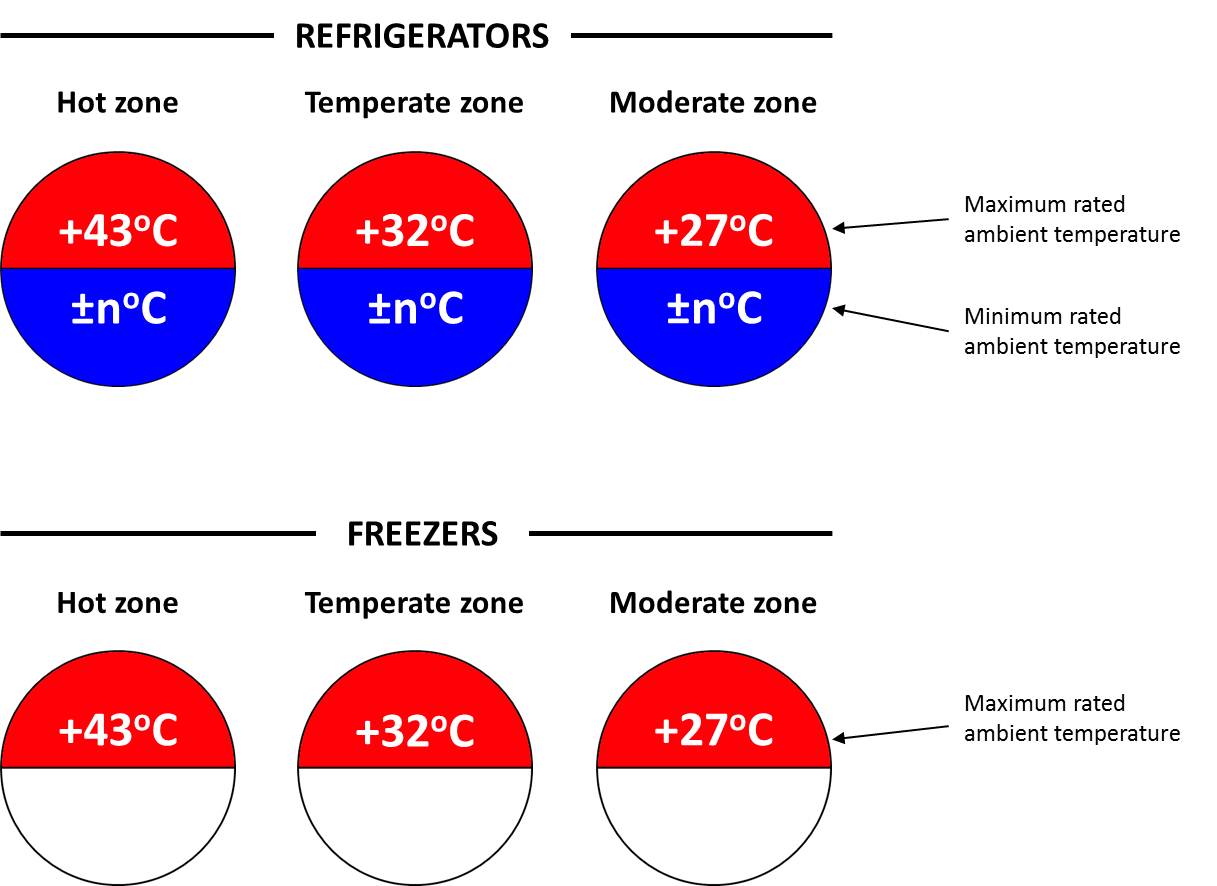
Temperature zone symbols for refrigerators: Circle shape signage indicating climate zones along with maximum and minimum rated temperatures for WHO PQS prequalified vaccine refrigerators and freezers.
For refrigerators, the upper semi-circle shows the maximum rated ambient temperature which if exposed to temperatures above this, the equipment will not maintain the vaccine at acceptable temperature range. The lower semi-circle shows the minimum rated ambient operating temperature that is established by PQS testing. If the equipment is exposed to temperatures below this figure, the vaccine will be at risk of freezing. The freezer symbols have a blank lower semi-circle because minimum rated ambient temperature is not relevant.
Temperature zone symbols for refrigerators (WHO)
Tertiary pack or carton: The pack or carton that contains a number of secondary cartons; usually constructed of corrugated fibreboard. The tertiary carton is not the same as the insulated shipper used for international air shipment of TTSPPs, although the insulated shipper may contain one or more of these cartons. (WHO)
Therapeutic equivalence: Two pharmaceutical products are considered to be therapeutically equivalent if they are pharmaceutically equivalent or pharmaceutical alternatives and after administration in the same molar dose, their effects, with respect to both efficacy and safety, are essentially the same when administered to patients by the same route under the conditions specified in the labelling. (WHO)
Thermal equilibrium: A state of no heat flow between two physical systems that are connected to each other through a path that is permeable to heat. Thermal equilibrium obeys the rules of zeroth law of thermodynamics. Thermal equilibrium also happens in one physical system when the temperature within the system is spatially and temporally uniform.
Thermal stability as lot release test: Stability of a vaccine after exposure to a temperature higher than that recommended for storage, for a specified period of time, often expressed in terms of change in potency. (WHO)
Thermal time constant: The most common definitions of the thermal reaction time are the so-called tau (τ, the 19th letter of the Greek alphabet) and "T90". Tau stands for the time a device needs to adapt to 63% of the end value of a temperature change whereas T90 represents the time to adapt to 90% of the change. T90 is approximately equal to 2.5 times Tau. These constants are commonly evaluated by experiment on the test device under well-defined conditions as described in EN 12830.
Thermistor: The term “thermistor” is used to describe an electronic component whose principle characteristic is a large change in electrical resistance with a change in its body temperature. The word “thermistor” is derived from the description “thermally sensitive resistor”. Thermistors used for precision temperature measurement are generally “negative temperature co-efficient” (NTC) types. NTC thermistors are devices whose resistance decrease as their temperature increases. NTC Thermistors offer many advantages in the area of temperature sensing: sensitivity (typically a -3% to -6% change in resistivity per a 1°C increase in temperature); small size, diverse configurations; and high accuracy. NTC Thermistors are manufactured from proprietary formulations of ceramic materials and transitional metal oxides (C Willis).
Thermocouple: Thermocouples are based on the change in the junction potential of two dissimilar metals as a function of temperature. Many metal pairs can be used, and each pair provides a unique range, accuracy, and precision. Precision and accuracy depend on the quality of the electronics used to measure the voltage across both metals and the type of temperature reference used. (USP 1118)
Thermolabile: Often used to describe biochemical substances which are subject to destruction/decomposition or change in response to heat.
Thermosensitivity: The state or condition of pharmaceutical/biological products being thermosensitive, showing reaction to heat. All vaccines are thermosensitive.
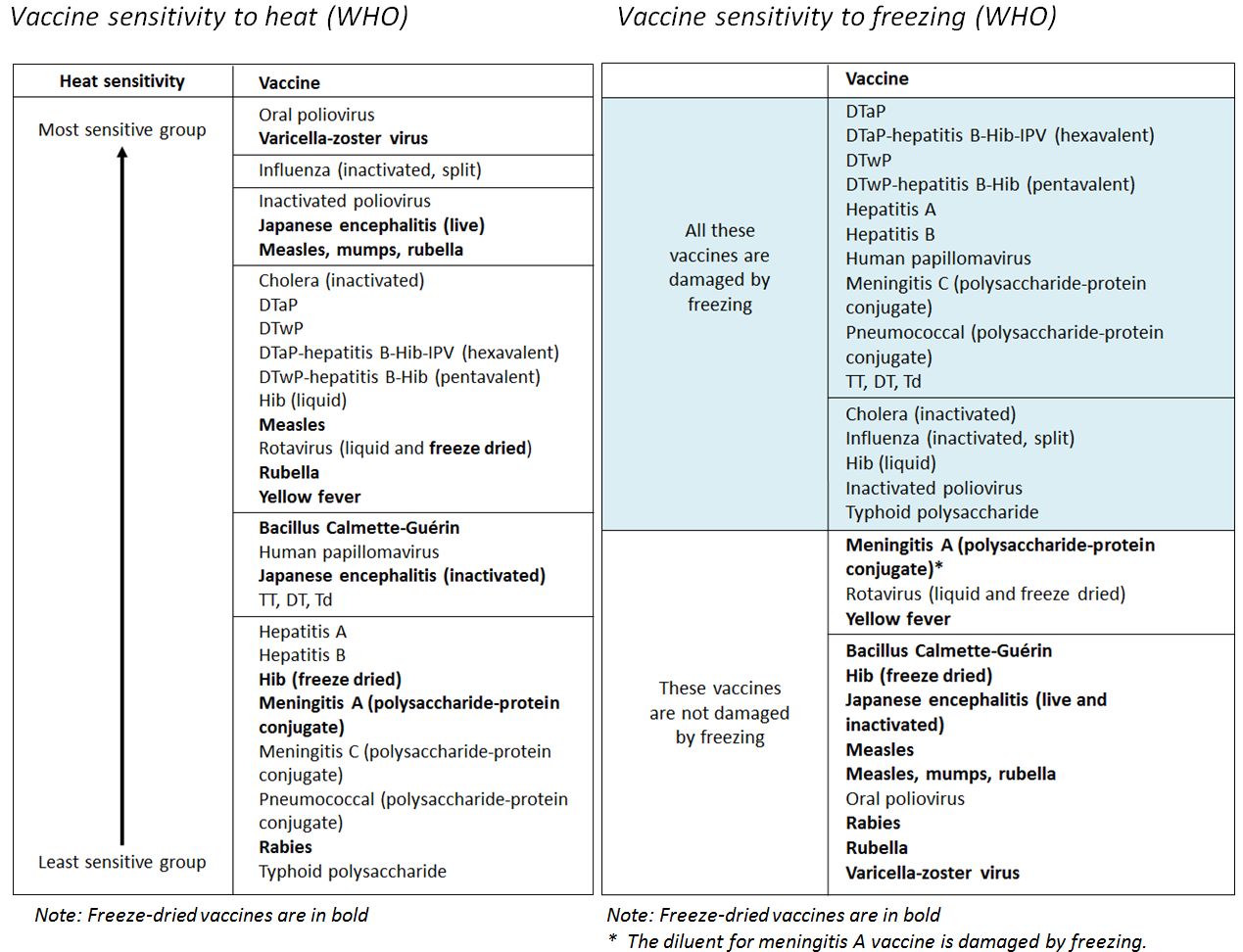
Thermostability: The quality of a substance to resist irreversible change in its chemical or physical structure at a high relative temperature.
Third-party accreditation: Accreditation or certification by an organization that issues credentials or certifies third parties against official standards as a means of establishing that a contractor is competent to undertake a specific type of work. Third-party accreditation organizations are themselves formally accredited by accreditation bodies; hence they are sometimes known as accredited certification bodies. The accreditation process ensures that their certification practices are acceptable, typically meaning that they are competent to test and certify third parties, behave ethically and employ suitable quality assurance.
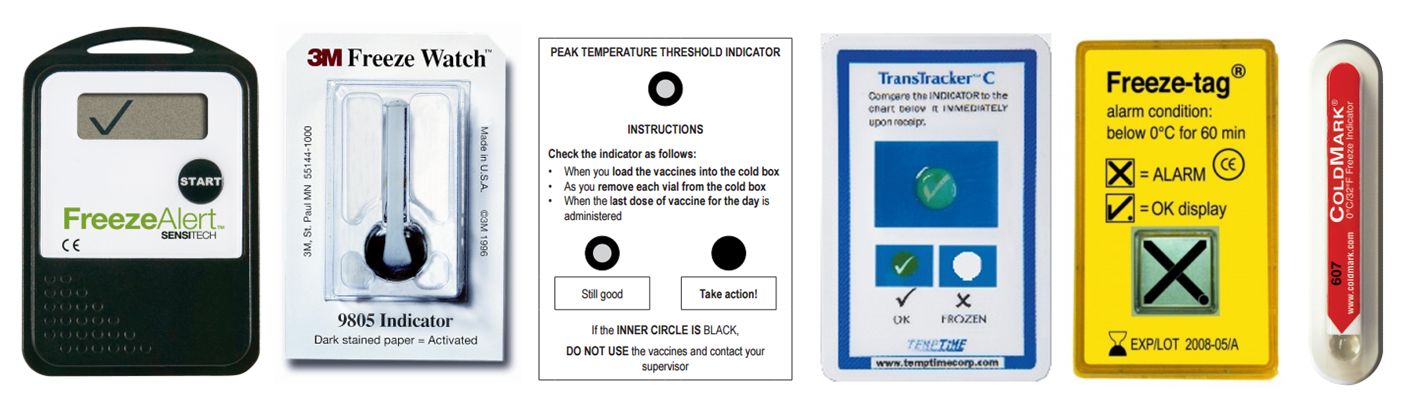
Threshold indicator: Indicators providing a signal only when exposed to temperatures higher than (ascending indicator) or lower than (descending indicator) a predetermined threshold temperature. Also see freeze indicator.
Examples of threshold indicators
Time and temperature sensitive label (IATA): A mandatory label for the transportation of healthcare cargo shipments. It is issued by the IATA and recognized by the air cargo industry as a best practice, and is effective since July 1st, 2012.
Time and temperature sensitive label (IATA)
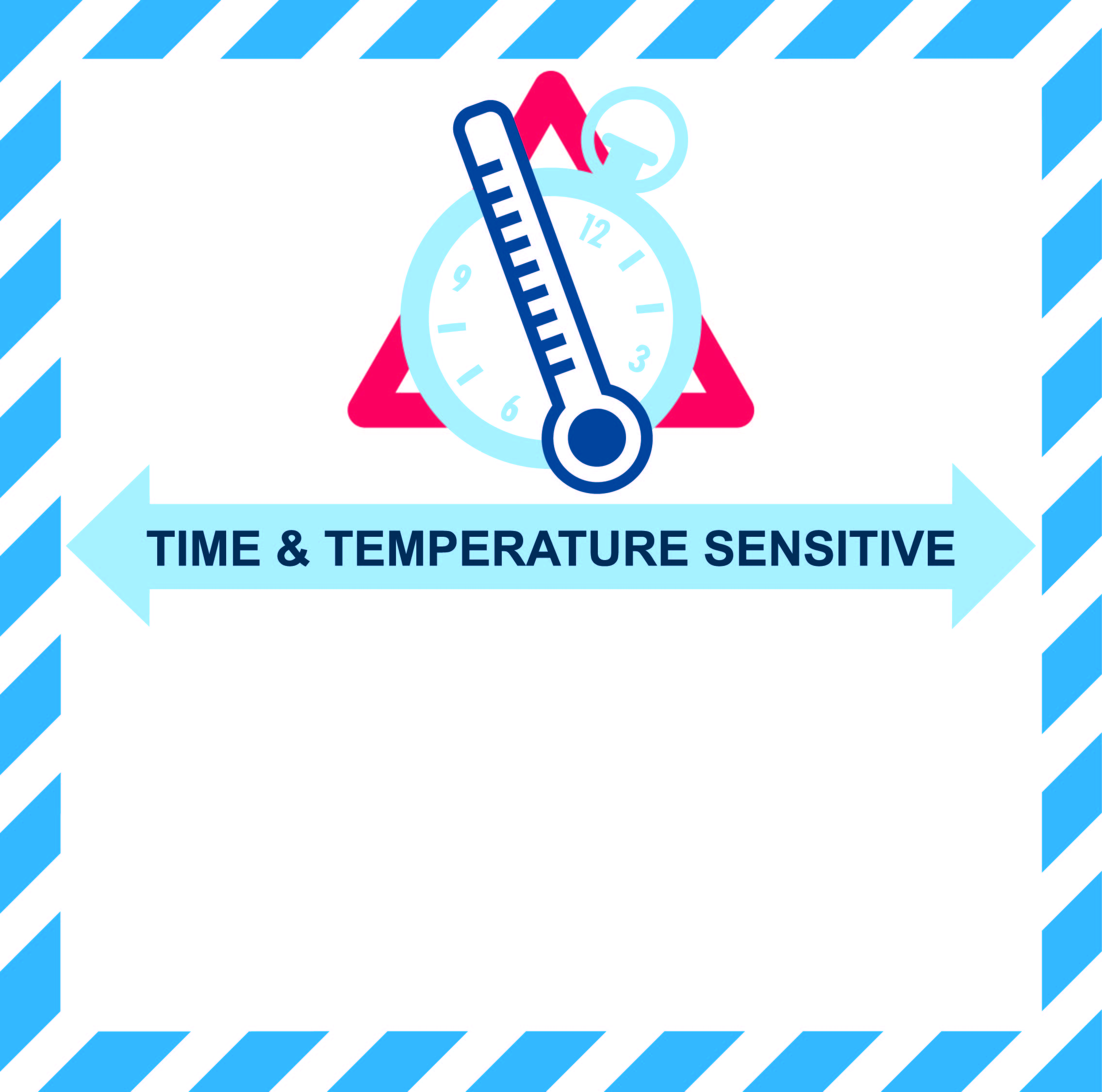
It is the responsibility of the shipper (or designated shipper’s agent by service agreement) to ensure the label is applied properly for time and temperature sensitive healthcare cargo shipments booked as such. The lower half of the label must never be left blank and must indicate the external transportation temperature range of the shipment. The temperature range must only be shown in Celsius. No other temperature information must be indicated on the label except, when agreed between the parties it may be used to communicate the Standard Operating Procedures (SOP) number. The temperature range indicated on the label always reflects the temperature external (or ambient temperature) to the package allowed during transportation and distribution and not the actual product (internal) temperature.
Time and temperature sensitive pharmaceutical product (TTSPP): Any pharmaceutical good or product which, when not stored or transported within predefined environmental conditions and/or within predefined time limits, is degraded to the extent that it no longer performs as originally intended. (WHO)
Time-temperature integrators (TTIs): Are generally chemically impregnated onto a pulp or paperboard substrate. Their reaction rate or diffusion process is used to estimate a temperature equivalent integrated over time. Thus, TTIs provide a measure of accumulated heat rather than instantaneous temperature such as a spike or critical threshold (see chemical indicators). The reactions are irreversible - once a color change, color development, or diffusion process has taken place, exposure to low temperatures will not restore the indicator to its original state. They change color, or are marked by a hue progression in intensity (generally from light to dark) in response to cumulative changes in temperature, such as heat, at a rate dependent on the Arrhenius equation. A TTI accumulates all of the temperature conditions experienced by the product to which it is affixed. The color development can be customized based on the known stability of the product, and in much the same way that most biologicals and pharmaceuticals degrade when exposed to heat - faster at higher temperatures, and slower at lower temperatures.
Traceability: The ability to identify and trace the history, distribution, location, and application of products, parts, materials, and services. A traceability system records and follows the trail as products, parts, materials, and services come from suppliers and are processed and ultimately distributed as final products and services. (ISO 9000:2015)
Transaction records: Records kept on products being moved from one facility to another. Also see stock keeping records and consumption records.
Transport temperature profile: Anticipated ambient temperature variation and duration to which a TTSPP may be exposed during transport. (WHO)
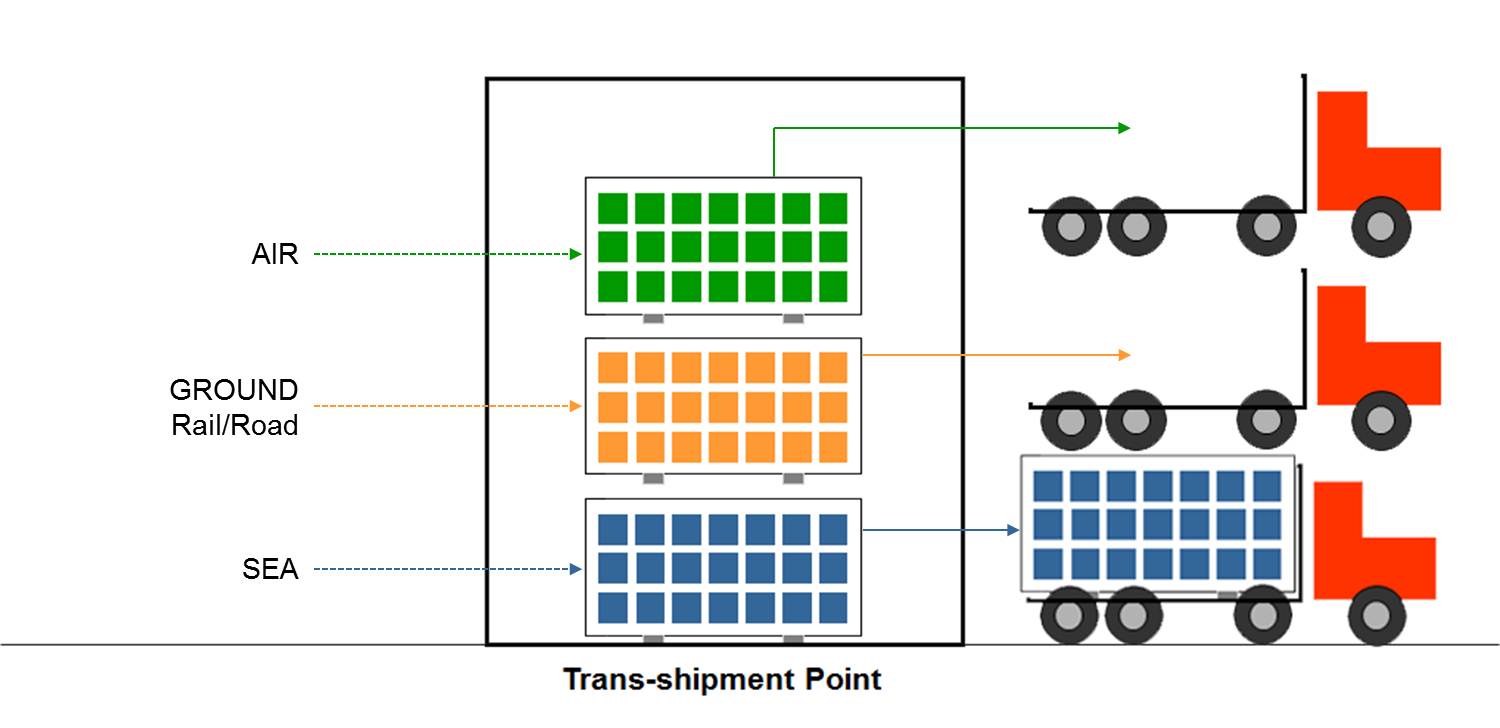
Trans-shipment point: Similar to cross-dock centres in that orders for individual customers are picked at the central location, transported in bulk in large vehicles to the trans-shipment point where they are split into individual orders for individual customers for delivery in small vehicles. (WHO)
Treaties: International agreements in which governments agree to follow particular standards or procedures. Also known as “conventions”. Most treaties are not immediately legally binding, but rather become binding only when a country’s internal government formally adopts the agreement. Many human rights principles have been established through international treaties, including the International Covenant on Civil and Political Rights, the International Covenant on Economic, Social, and Cultural Rights, and numerous regional human rights conventions. Human rights treaties are significant because they require governments to promote human rights in both the public and private sectors. In some cases, treaty-based obligations can be enforced in domestic courts or in international tribunals. (WHO)
Trial site: The location(s) where trial-related activities are actually conducted.
Trial subject: An individual who participates in a clinical trial, either as a recipient of the pharmaceutical product under investigation or as a control (WHO). The individual may be:
- a healthy person who volunteers to participate in a trial;
- a person with a condition unrelated to the use of the investigational product;
- a person (usually a patient) whose condition is relevant to the use of the investigational product.
Triple point: The temperature and pressure at which a substance can exist in equilibrium in the liquid, solid, and gaseous states. The triple point of pure water is at 0.01oC and 4.58 mm of mercury and is used to calibrate thermometers.
TT2+ coverage indicator: Percent of women who receive two and more TT vaccine in relation to the overall infants under one year of age or live births. The numerator is an aggregate of TT doses from the second dose onwards. The first dose is not included in the coverage indicator, but reported separately since it is not protective.
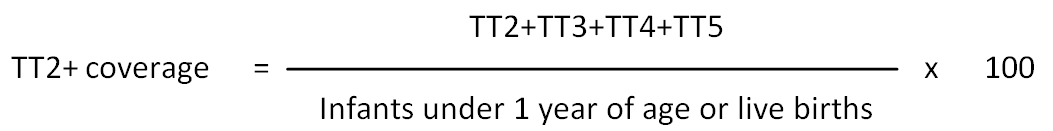
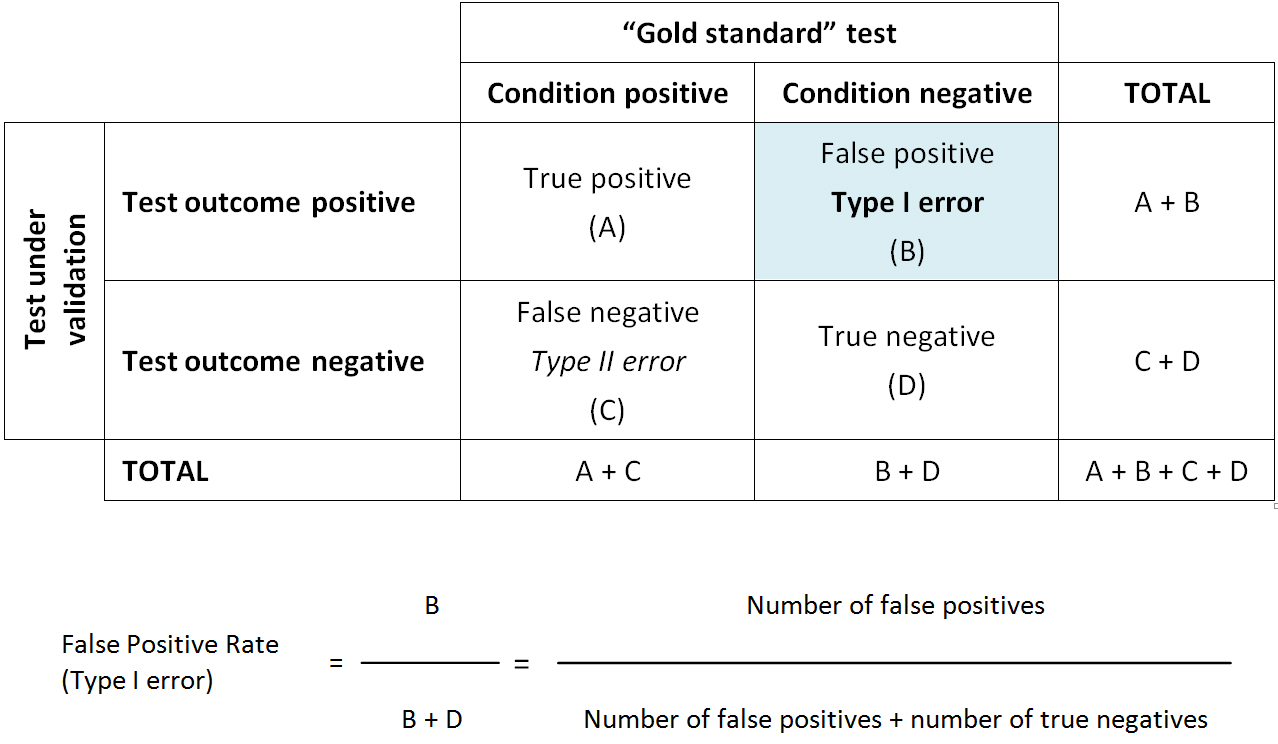
Type I error: The statistical error (said to be "of the first kind" or alpha error) made in testing a hypothesis when it is concluded that a treatment or intervention is effective when it really is not. Type I error is sometimes referred to as a “false positive”.
Type I error
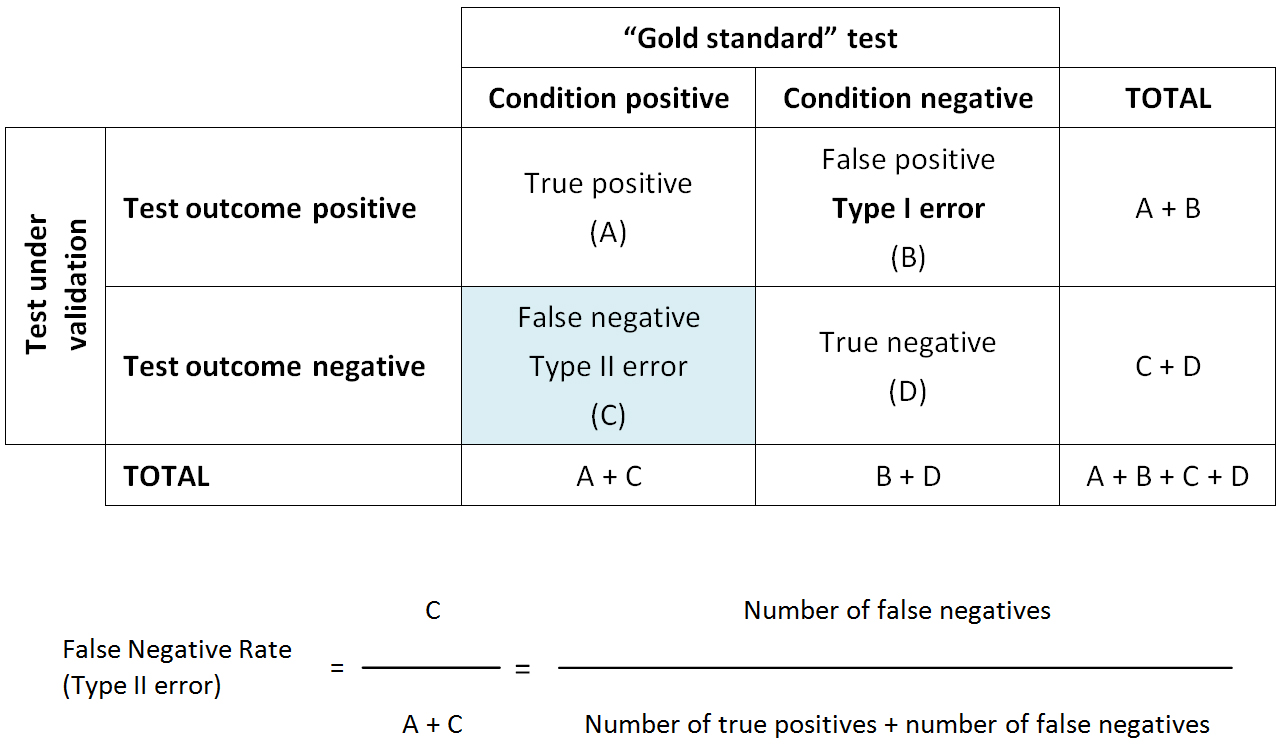
Type II error: The statistical error (said to be "of the second kind" or beta error) made in testing a hypothesis when it is concluded that a treatment or intervention is not effective when it really is. Type II error is sometimes referred to as a “false negative”.
Type II error
Type-examination: Some products such as cold rooms and solar power systems are site-specific but are made up of standard manufactured components. These are pre-qualified by a “type-examination” procedure. A technical expert carries out a detailed assessment against a standard checklist to determine that all the components comply fully with the specification. Type-examination is also used for certain other product types which meet normal industry standards, and are not considered to be programme-critical; for example, temperature data loggers used for temperature monitoring studies.
Type-testing: A product that is programme-critical is generally “type-tested”. Type-testing also starts with a “type-examination” procedure. This is followed by standardized laboratory testing to ensure full compliance with the critical performance requirements.

The users of this electronic publication are free to share (to copy, distribute, display and perform the work and make derivative works based on it only for noncommercial purposes); and to remix (to adapt the work) under the following conditions:
Attribution - The work must be attributed in the manner specified by the author or licensor (but not in a way that suggests that they endorse you or your use of work)