G
g-force: (gravitational force) A measurement of the type of acceleration that indirectly causes weight. When the g-force acceleration is produced by the surface of one object being pushed by the surface of another object, the reaction-force to this push produces an equal and opposite weight for every unit of an object’s mass. See damage indicator.
GAVI:  Created in 2000, GAVI is an international organization - a global Vaccine Alliance, based in Geneva, Switzerland, bringing together public and private sectors with the shared goal of creating equal access to new and underused vaccines for children living in the world’s poorest countries. For more information see http://www.gavi.org/
Created in 2000, GAVI is an international organization - a global Vaccine Alliance, based in Geneva, Switzerland, bringing together public and private sectors with the shared goal of creating equal access to new and underused vaccines for children living in the world’s poorest countries. For more information see http://www.gavi.org/
Generic products: The term generic product has somewhat different meanings in different jurisdictions. Use of this term is therefore avoided as much as possible, and the term multisource pharmaceutical product is used instead. Generic products may be marketed either under the approved non-proprietary name or under a brand (proprietary) name. They may be marketed in dosage forms and/or strengths different from those of the innovator products. Where the term generic product is used, it means a pharmaceutical product, usually intended to be interchangeable with the innovator product, which is usually manufactured without a licence from the innovator company and marketed after expiry of the patent or other exclusivity rights. The term should not be confused with generic names for APIs. (WHO)
Global Advisory Committee on Vaccine Safety (GACVS): A global standing advisory committee that provides independent, authoritative, scientific advice to WHO on vaccine safety issues of global or regional concern with the potential to affect in the short or long term national immunization programmes. This includes providing advice on urgent matters as needed. Issues to be considered by the Committee are jointly decided by the WHO Secretariat and the Committee. GACVS has 14 members, who serve in their personal capacity and represent a broad range of disciplines covering immunization activities. GACVS members are acknowledged experts from around the world selected from, but not necessarily restricted to, disciplines such as epidemiology, statistics, paediatrics, internal medicine, pharmacology and toxicology, infectious diseases, public health, immunology and autoimmunity, vaccinology, pathology, ethics, neurology, drug regulation and vaccine safety. (WHO)
Global Vaccine Safety Blueprint: A document published by the WHO to optimize the safety of vaccines through effective use of pharmacovigilance principles and methods. The blueprint strategic goals include:
- To assist low and middle income countries (LMIC) to have at least minimal capacity for vaccine safety activities.
- To enhance capacity for vaccine safety assessment in countries that introduce newly-developed vaccines, that introduce vaccines in settings with novel characteristics, or that both manufacture and use prequalified vaccines.
- To establish a global vaccine safety support structure.
The Global Vaccine Safety Blueprint is not the first document to draw attention to the need for improved global “vaccine pharmacovigilance.” Several organizations and partners, including WHO, UNICEF, GAVI and many international technical agencies, are involved in various aspects of vaccine pharmacovigilance either directly or indirectly. As a set of global strategies, however, the Global Vaccine Safety Blueprint represents an attempt to leverage international commitment and to set out a framework for coordinated action that will raise the level and accuracy of vaccine safety monitoring globally, and will enable cases of AEFI to be investigated wherever they occur.
Two major documents for the global vaccine safety (WHO)


A substantial proportion of AEFI results from inadequate handling and administration. Strategies to ensure safe programmatic usage of vaccines are devised by immunization programmes. They are not addressed in this document that focuses on the effects related, or believed to be related, to the vaccine product themselves. Separate guidance documents are available to address vaccine management and immunization techniques, in particular from WHO. For details see http://extranet.who.int/iris/restricted/bitstream/10665/70919/1/WHO_IVB_12.07_eng.pdf?ua=1 and http://apps.who.int/iris/bitstream/10665/70854/1/WHO_IVB_12.04_eng.pdf
Global Vaccine Safety Initiative (GVSI): WHO initiative launched in 2012, set up to implement the “Global Vaccine Safety Blueprint” strategy. The initiative comprises a framework of eight strategic objectives aimed at enhancing global vaccine safety activities. The strategic objectives focus on building and supporting a systemic approach to vaccine pharmacovigilance in all low and middle-income countries. The eight strategic objectives are:
- Strengthening vaccine safety monitoring in all countries.
- Strengthening the ability of countries to evaluate vaccine safety signals.
- Developing vaccine safety communication plans at country level to ensure awareness of vaccine risks and benefits, understand perceptions of risk, and prepare for managing any AEFI and crises promptly.
- Developing internationally harmonized tools and methods for vaccine pharmacovigilance.
- Advocating for the establishment of a legal, regulatory and administrative framework to ensure compliance with vaccine pharmacovigilance requirements at national, regional and international levels.
- Facilitating the strengthening of regional and global technical support platforms for a vaccine pharmacovigilance system that meets countries’ expressed needs.
- Making advice on vaccine safety issues available to support vaccine safety systems at national, regional and international levels.
- Facilitating the development of systems for appropriate interaction between national governments, multilateral agencies, and manufacturers at national, regional and international levels.
The GVSI is neither a legal entity, nor a partnership. It is a WHO mechanism for enhancing vaccine safety by providing a framework for WHO to convene its member states and partners for the implementation of the Global Vaccine Safety Blueprint. As a forum for collaboration between vaccine safety stakeholders, it highlights existing tools and resources, creates synergies, and prevents duplication of efforts and wasted resources towards the achievement of its mission. The work of the GVSI is supported by the WHO GVSI Secretariat.
Also see Global Vaccine Safety Blueprint.Globally Harmonized System of Classification and Labelling of Chemicals (GHS): An international agreed-upon system created by the United Nations, designed to harmonize and regulate classification and labelling standards of hazardous materials. GSH was created at the 1992 Rio Conference on Environment and Development.
Gold standard: A diagnostic test or benchmark that is the best available and current preferred method of diagnosing a particular condition (disease). All other methods available and new tests developed to diagnose the same condition should be validated against the gold standard. For example, “phase control microscopy” was used as a “golden test” for validating the “shake test” in diagnosing the freeze damaged aluminium adjuvanted vaccines. Also see validity.
Good clinical practice (GCP): An international ethical and scientific quality standard that is provided by ICH (E6/R1) for designing, conducting, recording and reporting trials that involve the participation of human subjects. Compliance with this standard provides public assurance that the rights, safety and well-being of trial subjects are protected, consistent with the principles that have their origin in the Declaration of Helsinki, and that the clinical trial data are credible. The guideline should be followed when generating clinical trial data that are intended to be submitted to regulatory authorities. The principles established in this guideline may also be applied to other clinical investigations that may have an impact on the safety and well-being of human subjects. ICH lists 13 principles for GCP:
- Clinical trials should be conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, and that are consistent with GCP and the applicable regulatory requirement(s).
- Before a trial is initiated, foreseeable risks and inconveniences should be weighed against the anticipated benefit for the individual trial subject and society. A trial should be initiated and continued only if the anticipated benefits justify the risks.
- The rights, safety, and well-being of the trial subjects are the most important considerations and should prevail over interests of science and society.
- The available nonclinical and clinical information on an investigational product should be adequate to support the proposed clinical trial.
- Clinical trials should be scientifically sound, and described in a clear, detailed protocol.
- A trial should be conducted in compliance with the protocol that has received prior institutional review board (IRB)/independent ethics committee (IEC) approval/favourable opinion.
- The medical care given to, and medical decisions made on behalf of, subjects should always be the responsibility of a qualified physician or, when appropriate, of a qualified dentist.
- Each individual involved in conducting a trial should be qualified by education, training, and experience to perform his or her respective task(s).
- Freely given informed consent should be obtained from every subject prior to clinical trial participation.
- All clinical trial information should be recorded, handled, and stored in a way that allows its accurate reporting, interpretation and verification.
- The confidentiality of records that could identify subjects should be protected, respecting the privacy and confidentiality rules in accordance with the applicable regulatory requirement(s).
- Investigational products should be manufactured, handled, and stored in accordance with applicable good manufacturing practice (GMP). They should be used in accordance with the approved protocol.
- Systems with procedures that assure the quality of every aspect of the trial should be implemented.
http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf
Good laboratory practice (GLP): A quality system concerned with the organizational process and the conditions under which non-clinical health and environmental safety studies are planned, performed, monitored, recorded, archived and reported. GLP principles may be considered as a set of criteria to be satisfied as a basis for ensuring the quality, reliability and integrity of studies, the reporting of verifiable conclusions and the traceability of data. (PDA)
Good regulatory practice (GRP): GRP aims to improve the efficiency and effectiveness of regulatory agencies and of regulators themselves (WHO). The elements of GRP for a regulatory agency may comprise, but are not limited to:
- Definition of the organization’s mission, vision and functions;
- Mechanisms to ensure that the organization is accountable to government, those regulated, and the public;
- Possibility to assess attainment of objectives;
- Mechanisms to ensure that outcomes are transparent to applicants, health professionals, and public;
- Commitment to equity;
- Arguments used to reach decision accessible to the public;
- Reasonable duration of assessment without compromising quality, safety & efficacy;
- Expedite review for orphan and outstanding public health- value medicines;
- Provisions for appeals and complaints;
- Ensuring that staff are:
- suitably qualified; and
- have the necessary facilities to perform their work at a high standard; and
- are appointed by a fair and transparent mechanism; and
- are of high integrity.
- Existence of a human resource development programme;
- Mechanisms for appeal and for citizens’ complaints;
- Access to appropriate knowledge and technology;
- Citizens are provided with accurate and appropriate medicines information;
- Mechanisms to ensure quality of operating procedures.
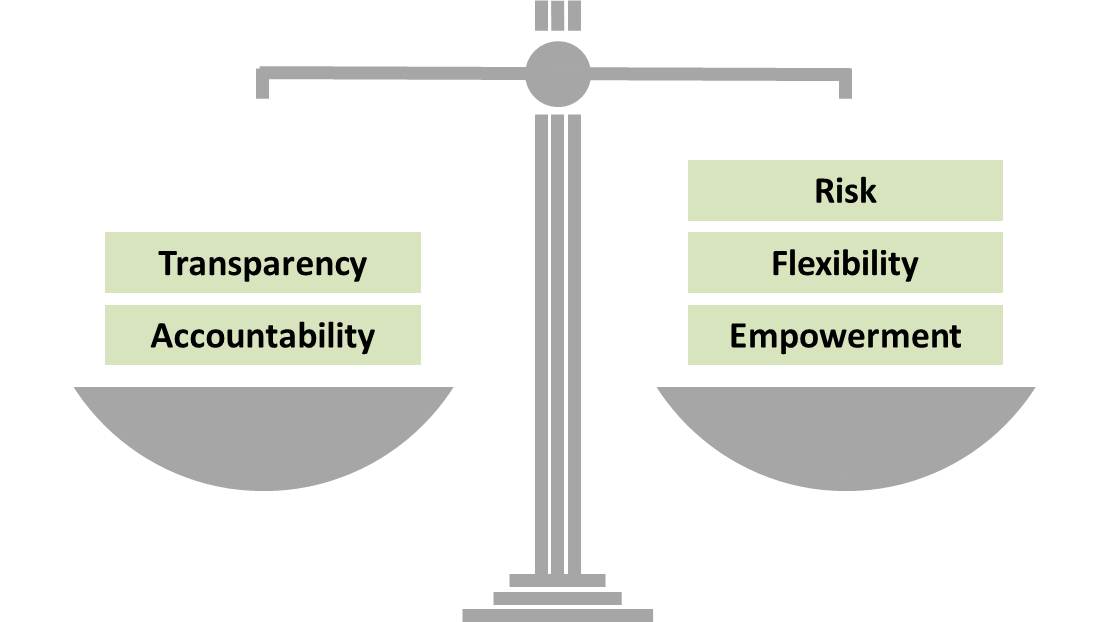
Modern management strategies involve giving more flexibility and responsibility to managers and staff at each level, and the ability to manage risk. This extra responsibility is sometimes referred to as empowerment. Consequently, in order to balance the extra power, there must be greater transparency and accountability.
Gross storage capacity: The gross free volume of a load support system available for storing SKUs. This volume is measured between the shelves of a shelving unit, or between the support beams of a racking system. (WHO)
Grossing factor: The actual internal volume of a cold room/freezer room, refrigerator or freezer, divided by the net volume of product that it can accommodate. (WHO)

The users of this electronic publication are free to share (to copy, distribute, display and perform the work and make derivative works based on it only for noncommercial purposes); and to remix (to adapt the work) under the following conditions:
Attribution - The work must be attributed in the manner specified by the author or licensor (but not in a way that suggests that they endorse you or your use of work)