R
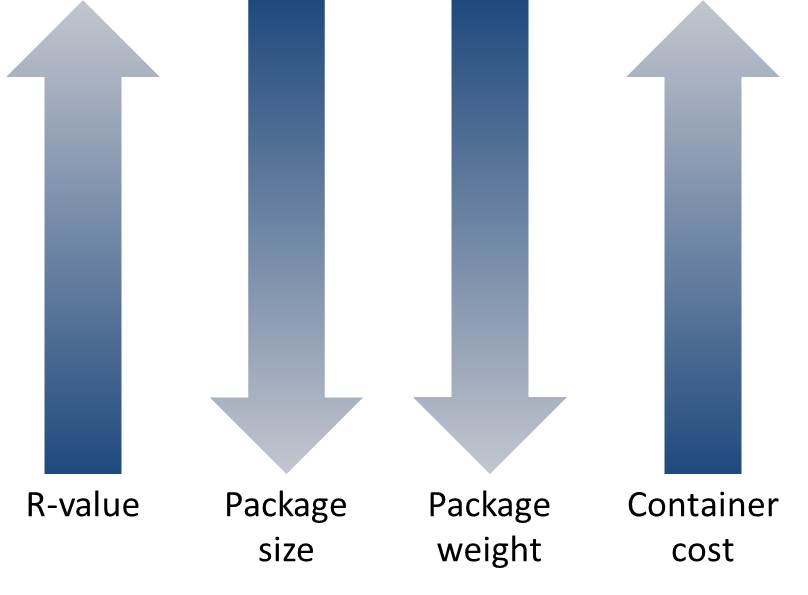
R-value (insulation): A measure of thermal resistance, expressed as the ratio of the temperature difference across an insulator and the heat flux (heat transfer per unit area per unit time, QA) through it or R=ΔT/QA. Although thermal resistance varies with temperature, it is usually treated as a constant value. The higher the value of R, the better is the insulation material’s theoretical effectiveness.
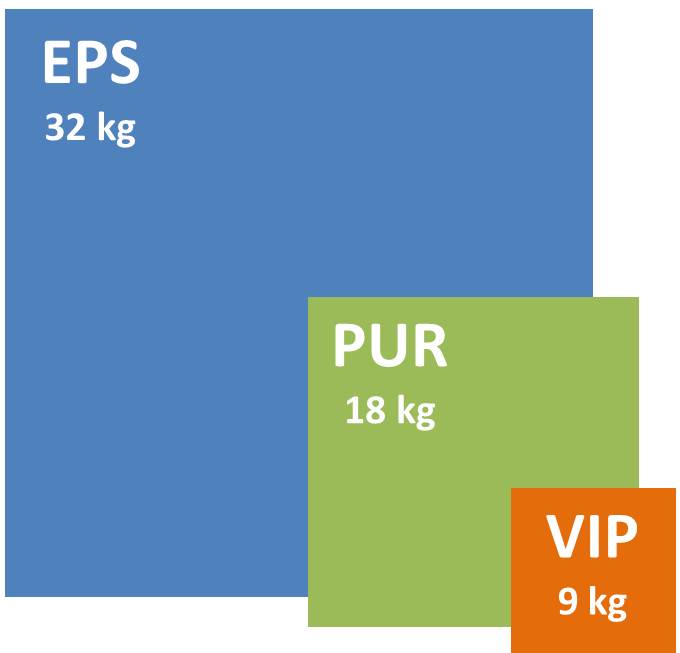
The impact of R-value on the packaging size for 1 litre of payload (Illustrated based on data from Thermosafe)
Radiation: Flow of atomic and subatomic particles and of waves, such as those that characterize heat rays, light rays, and X rays. Radiant energy from the sun warms the Earth.
RAG status: The red, amber or green color designation against pre-determined value range to break the risks into groups requiring different response strategies. Mostly used in two-axis risk score illustration like in PRA/PHA. See risk scales.
Random number: A number randomly selected.
Random sampling: A method in which chance alone determines who will be included in the sample, removing any possibility of selection bias.
Randomization: In its simplest form, randomization is a process by which n individuals are assigned to a test (nT) or control (nC) treatment so that all possible groups of size n = nT + nC have equal probability of occurring. Thus randomization avoids systematic bias in the assignment of treatment. It also promotes balance with respect to known and unknown prognostic factors that could affect the outcome of interest. While it does not guarantee that treatment groups will be exactly equal with respect to these factors, it does guarantee that any imbalance that occurs arose purely by chance. The process of randomization guarantees the validity of statistical analyzes of treatment effect, and (with adequate sample size) allows the detection, or ruling out, of small or moderate treatment differences. (WHO)
Rate: A ratio that expresses the frequency of a characteristic per 100 (or per 1000, per million, etc.) persons in the population at a given time.
Rate of consumption: The average quantity of stock dispensed to users during a particular time period.
Rational use of medicines: Rational use of medicines requires that "patients receive medications appropriate to their clinical needs, in doses that meet their own individual requirements, for an adequate period of time, and at the lowest cost to them and their community". Irrational use of medicines is a major problem worldwide. WHO estimates that more than half of all medicines are prescribed, dispensed or sold inappropriately, and that half of all patients fail to take them correctly. The overuse, underuse or misuse of medicines results in wastage of scarce resources and widespread health hazards. Examples of irrational use of medicines include: use of too many medicines per patient ("poly-pharmacy"); inappropriate use of antimicrobials, often in inadequate dosage, for non-bacterial infections; over-use of injections when oral formulations would be more appropriate; failure to prescribe in accordance with clinical guidelines; inappropriate self-medication, often of prescription-only medicines; non-adherence to dosing regimes. (WHO)
WHO advocates 12 key interventions to promote more rational use:
- Establishment of a multidisciplinary national body to coordinate policies on medicine use
- Use of clinical guidelines
- Development and use of national essential medicines list
- Establishment of drug and therapeutics committees in districts and hospitals
- Inclusion of problem-based pharmacotherapy training in undergraduate curricula
- Continuing in-service medical education as a licensure requirement
- Supervision, audit and feedback
- Use of independent information on medicines
- Public education about medicines
- Avoidance of perverse financial incentives
- Use of appropriate and enforced regulation
- Sufficient government expenditure to ensure availability of medicines and staff.
Raw data: All records or certified copies of original observations, clinical findings or other activities in a clinical trial necessary for the reconstruction and evaluation of the trial. Such material includes laboratory notes, memoranda, calculations and documents, as well as all records of data from automated instruments or exact, verified copies, e.g., in the form of photocopies or microfiches. Raw data can also include photographic negatives, microfilm, magnetic media (e.g., computer diskettes), optical media (CD-ROMs) and computer records. (WHO)
Reactogenicity: Reactions, either local or systemic, that are considered to have a causal relationship to the vaccination. (WHO)
Reagent: A substance other than a starting material, intermediate, or solvent that is used in the manufacture of a new drug substance. (ICH Q3A/R2)
Real-time, real-condition stability studies: Studies on the physical, chemical, biological, biopharmaceutical and microbiological characteristics of a vaccine, during and up to the expected shelf-life and storage periods of samples under the expected handling and storage conditions. The results are used to recommend storage conditions, and to establish the shelf-life and/or the release specifications. (WHO)
Recall: A process for withdrawing or removing a pharmaceutical material from the distribution chain because of defects in the materials or complaints of a serious nature. The recall might be initiated by the manufacturer/importer/distributor or a responsible agency. (WHO)
Receiving record: Transaction record that lists the names and quantities of items received. Usually paired with a packing slip.
Records: Records provide evidence that an event took place, an action was performed, or that a decision was made. Often, records are examined when something goes wrong, during an audit, or by a national authority during an inspection. It is essential that records are available and considered by the reader to be reliable and trustworthy. Records can be paper based, like logs or temperature charts, or electronic, like online graphs of temperature conditions.
Refrigerant: A substance or mixture used in a heat pump and refrigeration cycle. In most cycles the refrigerant undergoes phase transitions from liquid to gas or solid and back again.
Refrigerated container or reefer: A thermally insulated shipping container or intermodal freight container, equipped with an integrated refrigeration unit, used for the transport of TTSPPs, by road, rail or ocean freight. The refrigeration unit requires an external electrical power supply when located at a land based site, on a container ship or on a quay. During road transport electrical power is typically supplied by a diesel generator. (WHO)
A refrigerated container with front and back views (topae, Shutterstock)

Refrigerated vehicle: Road transport vehicle such as a van, truck or semi-trailer whose isolated thermostatically controlled cargo compartment is maintained at a temperature different (lower or higher) than the external ambient conditions. The environment inside the cargo compartment may be temperature-controlled or temperature-modified. (WHO)
Refrigeration equipment: The term “refrigeration” or “refrigeration equipment” means any equipment whose purpose is to lower air and product temperatures and/or to control relative humidity. (WHO)
Regulation: Written rules adopted by administrative agencies pursuant to authority granted to those agencies under applicable statutes. For example, many countries have statutes that give administrative agencies the authority to establish rules governing the approval of new medications. Pursuant to these statutes, agencies establish rules specifying the standards and procedures under which approval decisions will be made. In some countries, regulatory entities’ authority to inspect clinical trials derives from provisions of administrative regulations (WHO). For example, in 1987, the U.S. Food and Drug Administration, pursuant to authority granted to it by the U.S. Congress, adopted the following regulatory provision:
21 C.F.R. § 312.68 Inspection of investigator's records and reports.
An investigator shall upon request from any properly authorized officer or employee of FDA, at reasonable times, permit such officer or employee to have access to, and copy and verify any records or reports made by the investigator pursuant to § 312.62. The investigator is not required to divulge subject names unless the records of particular individuals require a more detailed study of the cases, or unless there is reason to believe that the records do not represent actual case studies, or do not represent actual results obtained.
Relabelling: The process of putting a new label on the material (see also labelling). (WHO)
Release specification: The combination of physical, chemical, biological, and microbiological tests and acceptance criteria that determine the suitability of an API or FPP at the time of its release. (WHO)
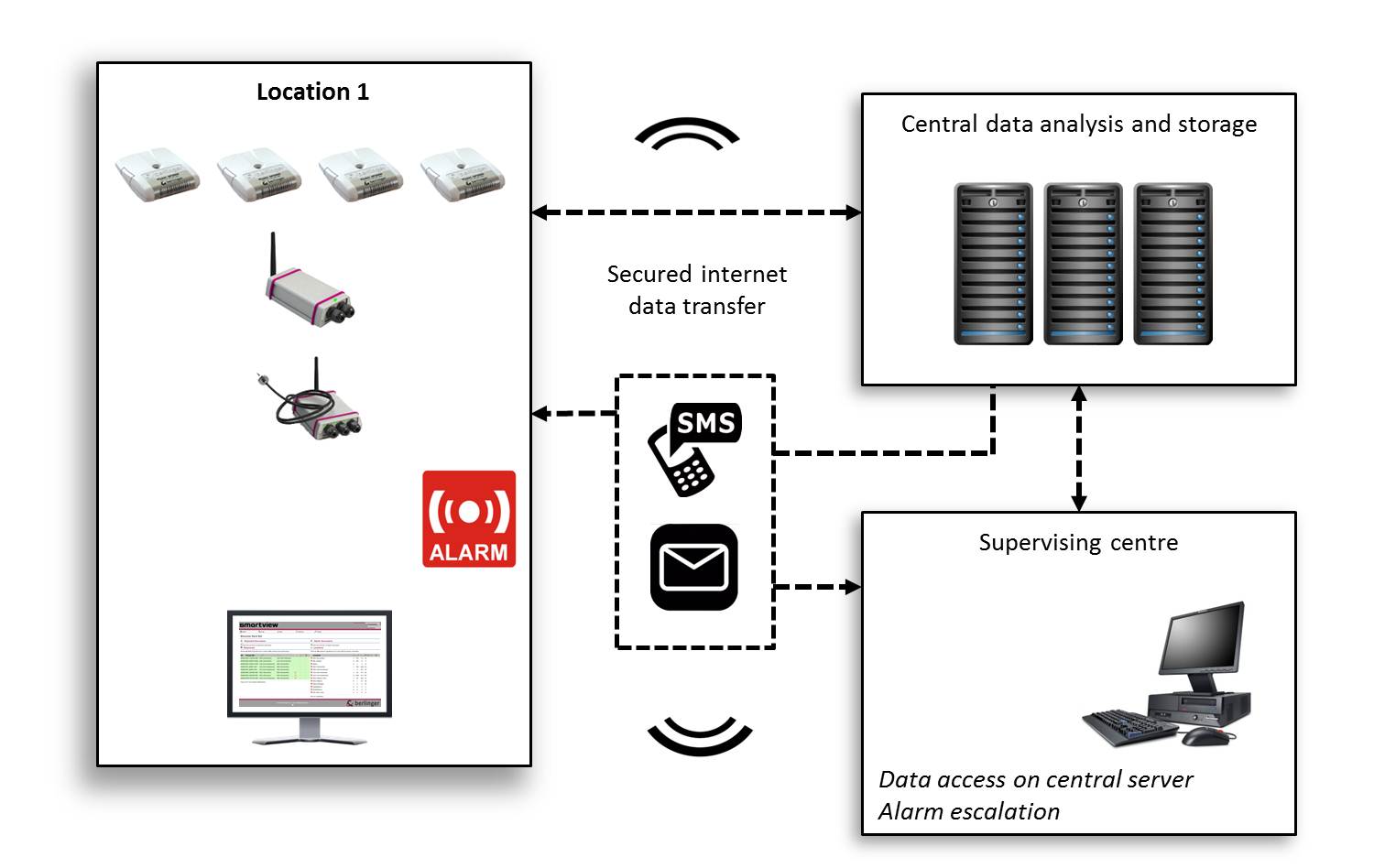
Remote temperature monitoring: Monitoring temperatures over an IP network.
A typical remote temperature monitoring system (Smartview/Berlinger & Co.)
Reorder level: See minimum stock level.
Repackaging: The action of changing the packaging of the material. (WHO)
Reproductive rate: The average number of secondary cases of an infection arising from a single primary case. The measure is inherent to the potential (infectiousness, susceptibility, measures of protection) of a microorganism to spread from person to person in a population. (WHO)
Request indicator: See minimum stock level.
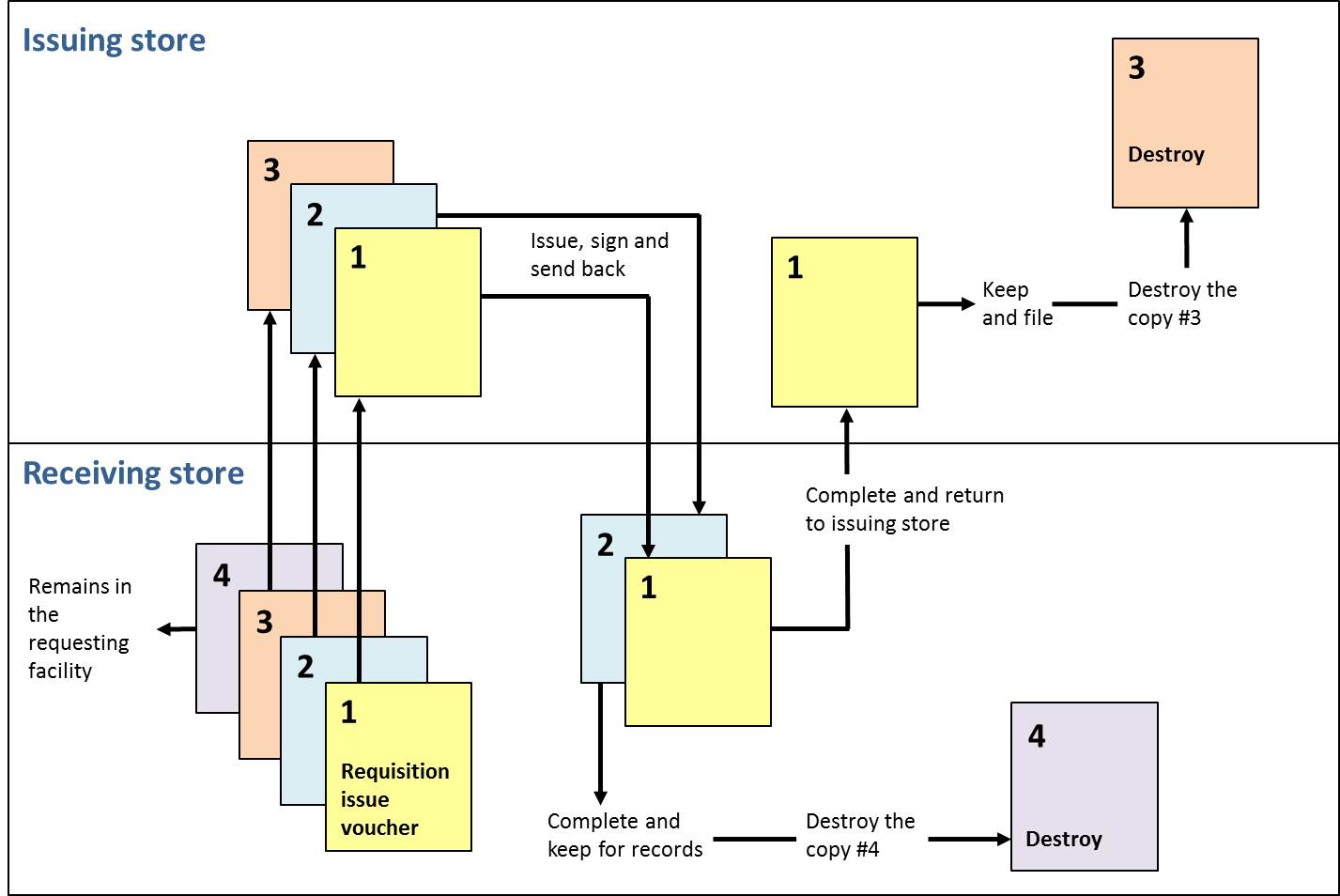
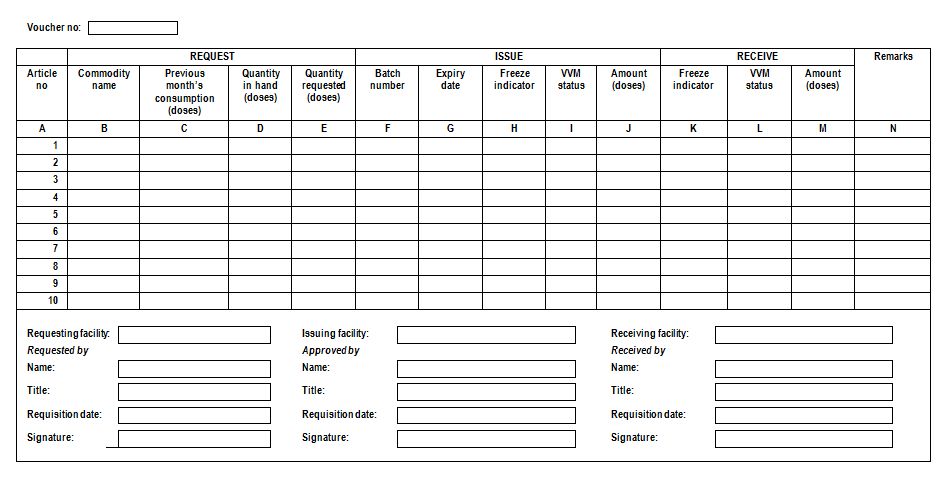
Requisition and issue voucher: Transaction record used in a pull distribution system that lists the items and quantities requested by a facility, the quantity actually issued and received. A requisition/issue voucher is similar to an issue voucher; however it is used in requisition/pull systems. Requisition/issue voucher lists the items and quantities requested by a facility. It also includes a column for the quantity actually issued. When issuing store supplies lesser quantity than requested, explanations should be given in remarks column. (WHO)
Requisition/issue voucher should be completed in four copies. The requesting facility completes the form, signs the record and sends the top three copies (1, 2, and 3) to the issuing facility, keeping the bottom copy (4). The issuing facility fills in the order, signs the form, and sends the top two copies (1 and 2) to the receiving facility, along with the supplies, keeping the bottom copy (3) as a reminder. Upon arrival of goods, the receiving facility verifies the quantity received, signs the form and sends back the top copy (1). The receiving facility keeps the second copy (2) for its files and destroys the copy (4) that was kept before. The top copy (1) arrives at the issuing facility, which then issuing facility destroys the reminder (3) and keeps the top copy (1) for its files. At the end, each of the facilities ends up with a completed copy of requisition/issue voucher for filing. Below Figure illustrates the flow of a requisition/ issue voucher.
Flow diagram for requisition and issue voucher (Kartoglu)
Sample requisition and issue voucher (WHO)
Requisition system: See pull system.
Research participant: An individual who participates in a biomedical research project, either as the direct recipient of an intervention (e.g., study product or invasive procedure), as a control, or through observation. The individual may be a healthy person who volunteers to participate in the research, or a person with a condition unrelated to the research carried out who volunteers to participate, or a person (usually a patient) whose condition is relevant to the use of the study product or questions being investigated. (WHO)
Reseller: A reseller is a commercial entity, licensed to act on behalf of a Legal Manufacturer, and which carries product liability and warranty responsibilities no less onerous than those carried by the Legal Manufacturer. (WHO)
Residual risk: Risk remaining after all control measures have been taken. It’s the risk remaining after you’ve reduced the risk, removed the source of the risk, modified the consequences, changed the probabilities, transferred the risk, or retained the risk.
Re-test date: The date after which an active API should be re-examined to ensure that the material is still in compliance with the specification and thus is still suitable for use in the manufacture of an FPP. (WHO)
Re-test period: The period of time during which the API is expected to remain within its specification and, therefore, can be used in the manufacture of a given FPP, provided that the API has been stored under the defined conditions. After this period a batch of API destined for use in the manufacture of an FPP should be re-tested for compliance with the specification and then used immediately. A batch of API can be re-tested multiple times and a different portion of the batch used after each re-test, as long as it continues to comply with the specification. For most substances known to be labile, it is more appropriate to establish a shelf-life than a re-test period. The same may be true for certain antibiotics. (WHO)
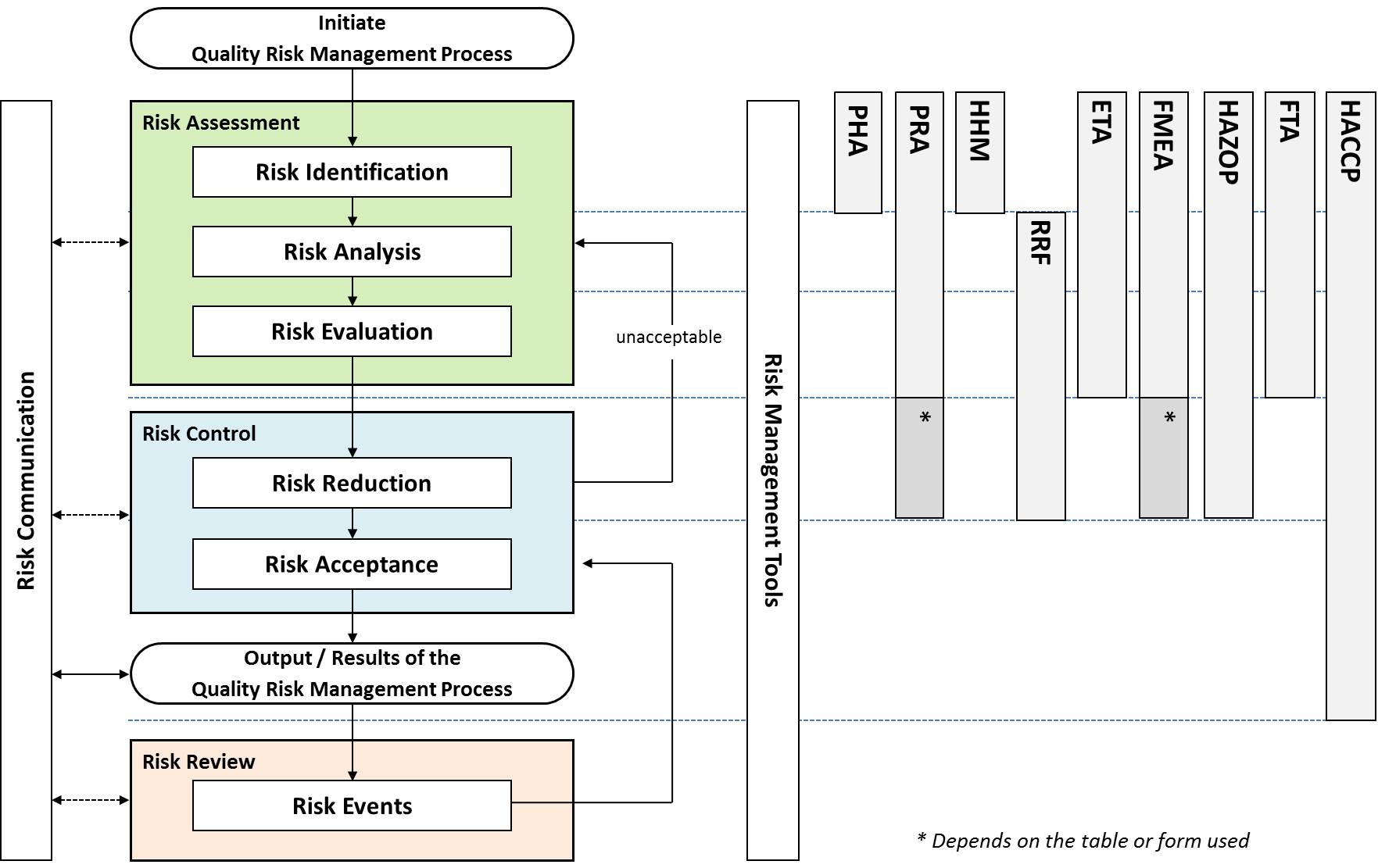
Risk: The combination of the probability of occurrence of harm and the severity of that harm (ISO 14971:2000).
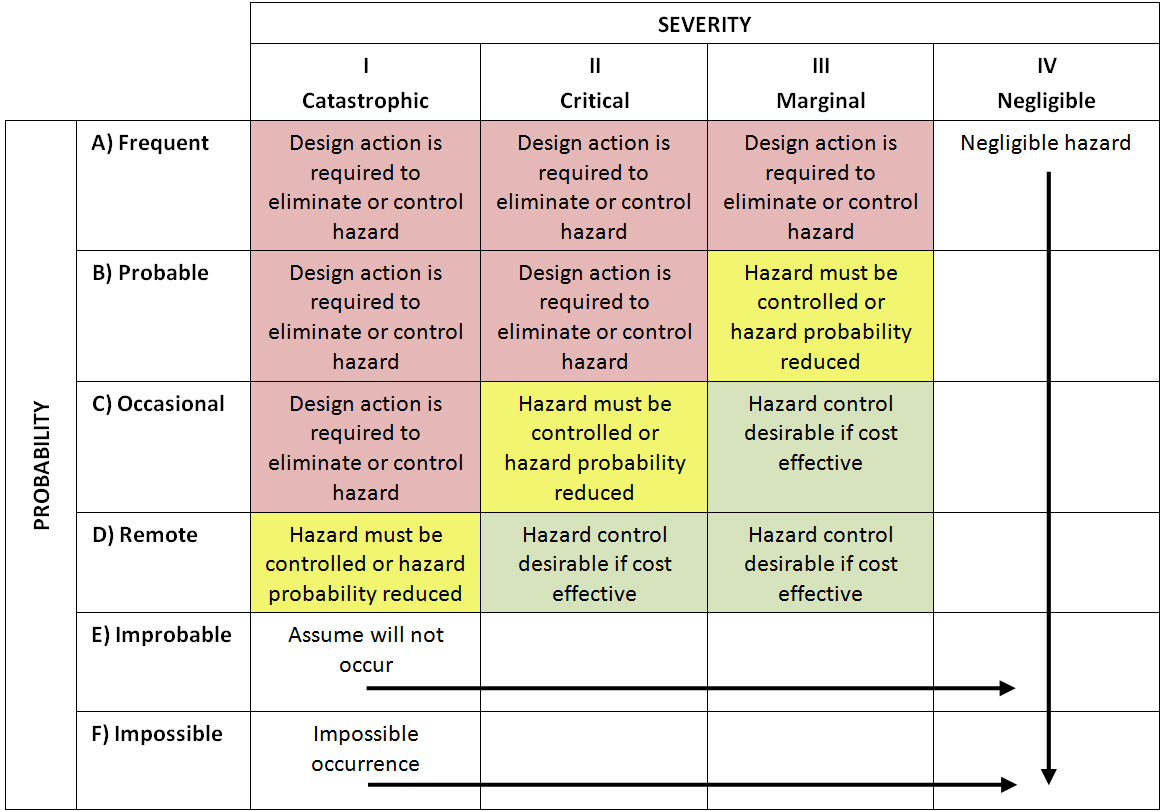
Risk analysis: The estimation of the risk associated with the identified hazards. It is the qualitative or quantitative process of linking the likelihood of occurrence and severity of harms. In some risk management tools, the ability to detect the harm (detectability) also factors in the estimation of risk. (ICH Q9)
Risk assessment: A systematic process of organizing information to support a risk decision to be made within a risk management process. It consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards. Quality risk assessments begin with a well-defined problem description or risk question. When the risk in question is well defined, an appropriate risk management tool and the types of information needed to address the risk question will be more readily identifiable. (ICH Q9)
As an aid to clearly defining the risk(s) for risk assessment purposes, three fundamental questions are often helpful:
- What might go wrong?
- What is the likelihood (probability) it will go wrong?
- What are the consequences (severity)?
Risk communication: The sharing of information about risk and risk management between the decision maker and other stakeholders. (ICH Q9)
Risk control: Actions implementing risk management decisions. (ISO Guide 73)
Risk evaluation: The comparison of the estimated risk to given risk criteria using a quantitative or qualitative scale to determine the significance of the risk. (ICH Q9)
Risk control might focus on the following questions:- Is the risk above an acceptable level?
- What can be done to reduce or eliminate risks?
- What is the appropriate balance among benefits, risks and resources?
- Are new risks introduced as a result of the identified risks being controlled?
Risk identification: The systematic use of information to identify potential sources of harm (hazards) referring to the risk question or problem description. Risk identification addresses the “What might go wrong?” question, including identifying the possible consequences. This provides the basis for further steps in the quality risk management process. (ICH Q9)
Risk management: The systematic application of quality management policies, procedures, and practices to the tasks of assessing, controlling, communicating and reviewing risk. (ICH Q9)
Risk management framework: A set of components that support and sustain risk management throughout an organization (ISO 31000). In risk management framework there are two types of components: foundations and organizational arrangements. Foundations include risk management policy, objectives, mandate, and commitment. And organizational arrangements include the plans, relationships, accountabilities, resources, processes, and activities used to manage organization’s risk.
Risk management process: One that systematically applies management policies, procedures, and practices to a set of activities intended to establish the context, communicate and consult with stakeholders, and identify, analyze, evaluate, treat, monitor, and review risk. (ISO 31000)
Risk matrix: A matrix that is used during risk assessment to define the various levels of risk as the product of the harm probability categories and harm severity categories. The two-criterion model of severity and probability based matrix is a typical risk matrix. See risk scales.
Example of two-factor risk matrix
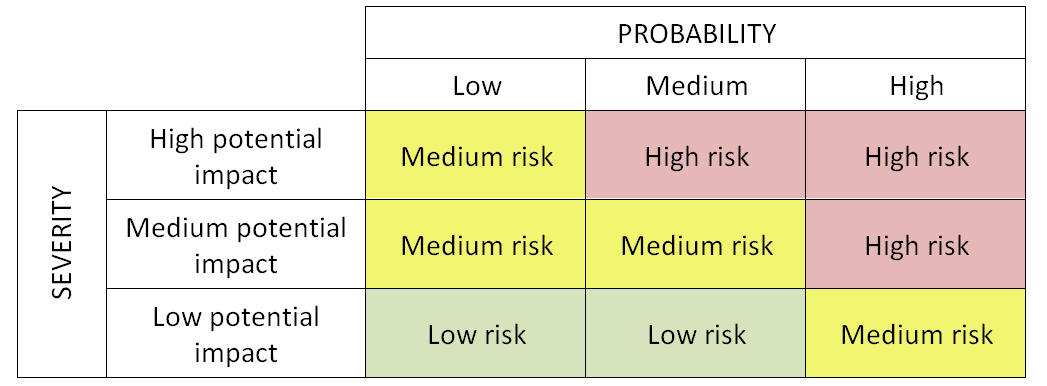
Tabular representation of risk levels (J Vesper)
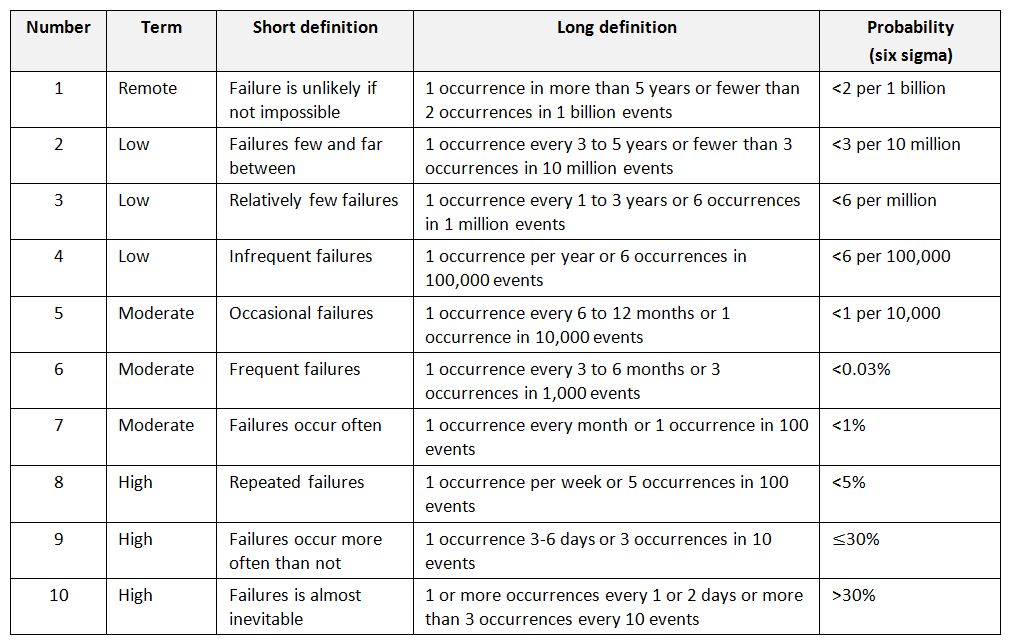
For likelihood (probability), the move has been to have terms that are more “observable” or “countable”.
An example of occurrence scale (adapted by J Vesper)
Risk ranking and filtering (RRF): Risk ranking and filtering is a tool for comparing and ranking risks. Risk ranking of complex systems typically requires evaluation of multiple diverse quantitative and qualitative factors for each risk. The tool involves breaking down a basic risk question into as many components as needed to capture factors involved in the risk. These factors are combined into a single relative risk score that can then be used for ranking risks. “Filters,” in the form of weighting factors or cut-offs for risk scores, can be used to scale or fit the risk ranking to management or policy objectives. (ICH Q9)
Risk ranking and filtering can be used to develop standardized risk rating forms prioritize vendor or contractor sites for inspection/audit by regulators or industry. Risk ranking methods are particularly helpful in situations in which the portfolio of risks and the underlying consequences to be managed are diverse and difficult to compare using a single tool. Risk ranking is useful when management needs to evaluate both quantitatively-assessed and qualitatively-assessed risks within the same organizational framework. Limitations of RRF include that only risks included in the filtering get examined and that creating risk rating forms takes significant time of subject matter experts.
Risk reduction: Actions taken to lessen the probability of occurrence of harm and the severity of that harm. (ICH Q9). See also risk treatment.
Risk review: Review or monitoring of output/results of the risk management process considering (if appropriate) new knowledge and experience about the risk. (ICH Q9)
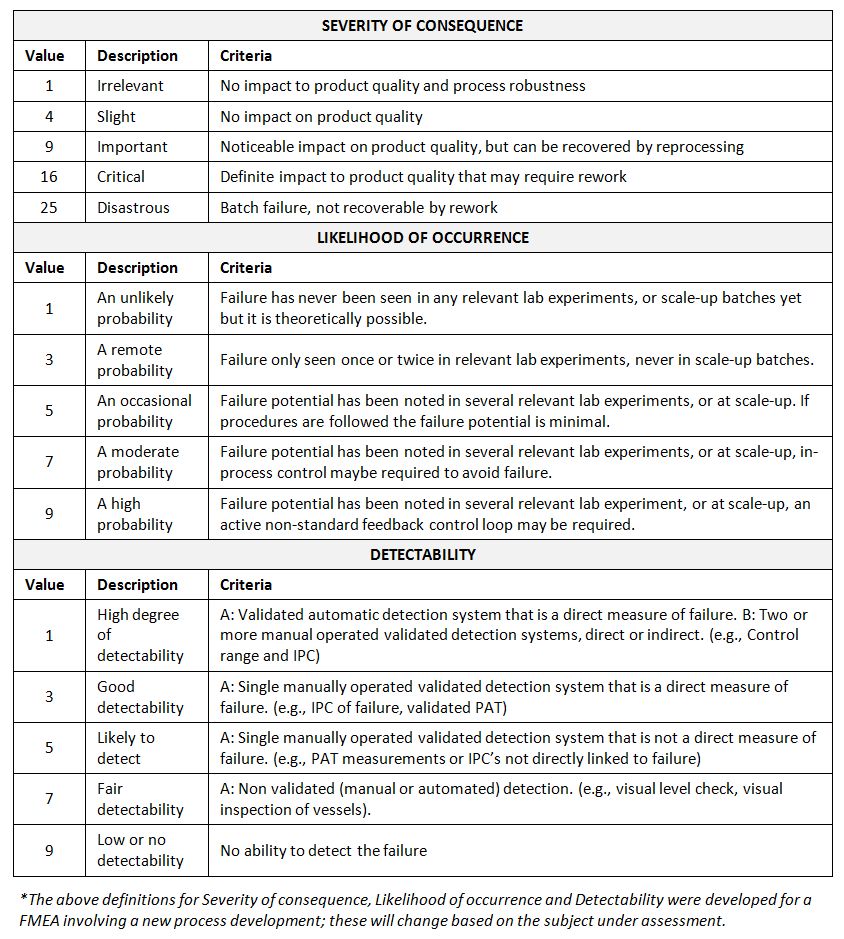
Risk scales (used in risk assessment): As for quantitative risk assessment tools, risk scales used for rating the severity of consequence, likelihood of occurrence and detectability should be clearly articulated. This is important to appropriate levels of differentiation in order to support meaningful risk prioritization. It also helps for consistency across the risk profile attributes to ensure that all attributes proportionally contribute to the overall prioritization of the risk. Risk scales (definitions, levels and numbers) may vary depending on the system under evaluation.
In order to appropriate levels of differentiation, five-point rating scales are widely used. Use of non-consecutive numbers (e.g., 1, 3, 5, 7, 10) are more useful compared to use of consecutive numbers (e.g., 1, 2, 3, 4, 5) in scales since non-consecutive numbers are proven to be less useful in differentiation. In the case of the desire to put more emphasis on the severity criteria, alternative a non-linear scoring scale can be utilized (e.g., 1, 4, 9, 16, 25).
Examples of risk scales for FMEA* (modified from PQRI)
As for PRA/PHA where in general likelihood of occurrence and severity of consequence are rated and risk score is calculated based on these two criteria, response strategies can be illustrated using a matrix and traffic light system by assigning red, amber or green against pre-determined value range. This breaks the risks into groups requiring different response strategies. This color designation is also known as “RAG status”.
As same linear scales can be used for both likelihood of occurrence and severity of consequence, a doubled risk scale may be used especially for severity element as it gives more weight to risks with a high impact. A risk with a low probability but a high impact is thus viewed as much more severe than a risk with a high probability and a low impact. This avoids any averaging out of serious risks.
Linear and doubled risk scales used in PRA/PHA
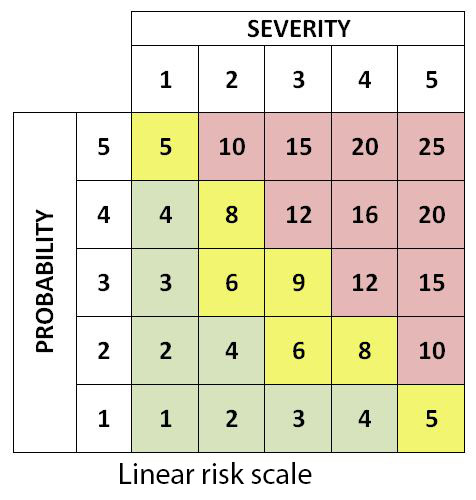
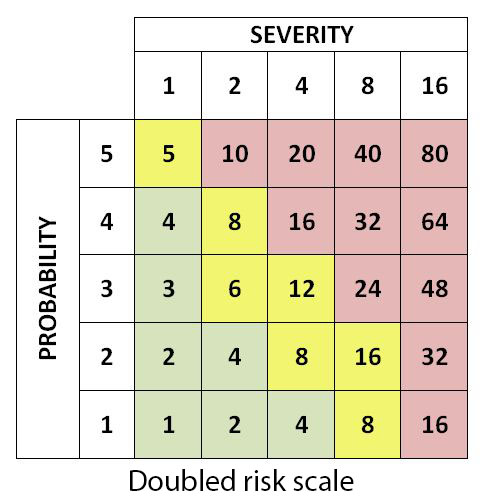
In this regard, red risks are unacceptable and have the priority in response strategy. Amber risks are moderate risks having secondary priority while green risks are acceptable (but this does not mean they can be ignored - they should be addressed at least through means of contingency). See also risk matrix.
Risk treatment: The process of selecting and implementing of measures to modify risk. Risk treatment measures can include avoiding, reducing, optimizing, transferring or retaining risk. Once the treatment is being implemented, it becomes a control and/or it modifies existing controls. ISO 31000:2009 gives a list on how to deal with risk:
- Avoiding the risk by deciding not to start or continue with the activity that gives rise to the risk
- Accepting or increasing the risk in order to pursue an opportunity
- Removing the risk source
- Changing the likelihood
- Changing the consequences
- Sharing the risk with another party or parties (including contracts and risk financing)
- Retaining the risk by informed decision
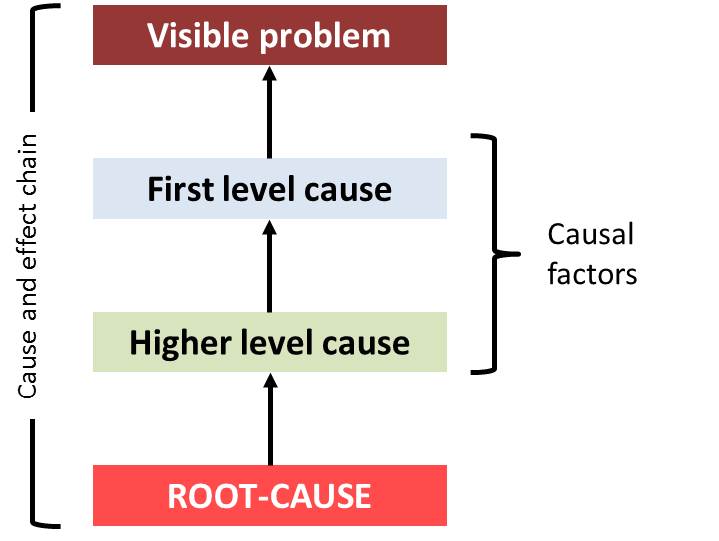
Root-cause: A factor considered if removal thereof from the problem-fault-sequence prevents the final unwanted event from recurring. Root-cause is the “evil at the bottom” setting in motion the whole cause and effect chain.
Root-cause analysis: A collective term that describes a wide range of approaches, tools, and techniques used to uncover the root causes or faults or problems. Root cause analysis must be performed systematically, usually as part of an investigation, with conclusions and root causes that are identified with documented evidence, which requires typically a team effort. There might be more than one root-cause for an unwanted event. The root cause(s) identified depends on the way in which the visible problem is defined. Effective problem statements and descriptions are important to ensure the execution of appropriate analyzes. There are various approaches that can be used in root-cause analysis: such as 5 whys, appreciation (so what technique), drill-down, cause and effect diagrams (fish-bone diagram, fault tree analysis), and failure mode effects analysis.
Route of administration: The means by which the candidate [vaccine] product is introduced to the host. Possible routes of administration include the intravenous, intramuscular, subcutaneous, transcutaneous, intradermal, transdermal, oral, intranasal, intranodal, intravaginal and intrarectal routes. (WHO)

The users of this electronic publication are free to share (to copy, distribute, display and perform the work and make derivative works based on it only for noncommercial purposes); and to remix (to adapt the work) under the following conditions:
Attribution - The work must be attributed in the manner specified by the author or licensor (but not in a way that suggests that they endorse you or your use of work)