Q
Qualification: Action of proving that any premises, equipment and supporting systems work correctly and actually lead to the expected results. The meaning of the word validation is sometimes extended to incorporate the concept of qualification. (WHO)
Qualification protocol: A written and approved plan detailing how a qualification will be conducted including test parameters, product characteristics, equipment and acceptance criteria. (WHO)
Qualified person (QP): The regulations specify that no batch of medicinal product can be released for sale or supply prior to certification by a QP that the batch is in accordance with the relevant requirements. The QP is typically a licensed pharmacist, biologist or chemist (or a person with another permitted academic qualification) who has several years of experience working in pharmaceutical manufacturing operations, and has passed examinations attesting to his or her knowledge. The requirement for QP oversight has been extended to material for use in clinical trials since the introduction of EU Directive 2001/20/EC. In countries that are part of PIC/S, “responsible person” and/or “authorized person” terms are used interchangeably.
Qualified third party: An entity independent from the company that is mandated and involved in the preparation, execution or analysis of a quality assurance (QA) activity for the company. This third party should present the adequate professional qualification to perform QA activities. (WHO)
Quality: The suitability of either a drug substance or a drug product for its intended use. This term includes such attributes as the identity, strength, and purity (ICH Q6A).
Quality agreement: See service level agreement.
Quality assurance: A wide-ranging concept covering all matters that individually or collectively influence the quality of a product. It is the totality of the arrangements made with the object of ensuring that pharmaceutical products are of the quality required for their intended use. Quality assurance therefore incorporates GMP and other factors, including those outside the scope of this guide such as product design and development. (WHO)
The system of quality assurance appropriate to the manufacture of pharmaceutical products should ensure that (WHO):
- Pharmaceutical products are designed and developed in a way that takes account of the requirements of GMP and other associated codes such as those of good laboratory practice (GLP) and good clinical practice (GCP);
- Production and control operations are clearly specified in a written form and GMP requirements are adopted;
- Managerial responsibilities are clearly specified in job descriptions;
- Arrangements are made for the manufacture, supply and use of the correct starting and packaging materials;
- All necessary controls on starting materials, intermediate products, and bulk products and other in-process controls, calibrations, and validations are carried out;
- The finished product is correctly processed and checked, according to the defined procedures;
- Pharmaceutical products are not sold or supplied before the authorized persons have certified that each production batch has been produced and controlled in accordance with the requirements of the marketing authorization and any other regulations relevant to the production, control and release of pharmaceutical products;
- Satisfactory arrangements exist to ensure, as far as possible, that the pharmaceutical products are stored by the manufacturer, distributed, and subsequently handled so that quality is maintained throughout their shelf-life;
- There is a procedure for self-inspection and/or quality audit that regularly appraises the effectiveness and applicability of the quality assurance system;
- Deviations are reported, investigated and recorded;
- There is a system for approving changes that may have an impact on product quality;
- Regular evaluations of the quality of pharmaceutical products should be conducted with the objective of verifying the consistency of the process and ensuring its continuous improvement.
Quality audit: An audit that consists of an examination and assessment of all or part of a quality system with the specific purpose of improving it. A quality audit is usually conducted by outside or independent specialists or a team designated by the management for this purpose. Such audits may also be extended to suppliers and contractors. (WHO)
Quality by design (QbD): A systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management. (ICH Q8/R2)
Quality control (QC): The operational techniques and activities undertaken within the quality assurance system to verify that the requirements for quality of the product have been fulfilled.
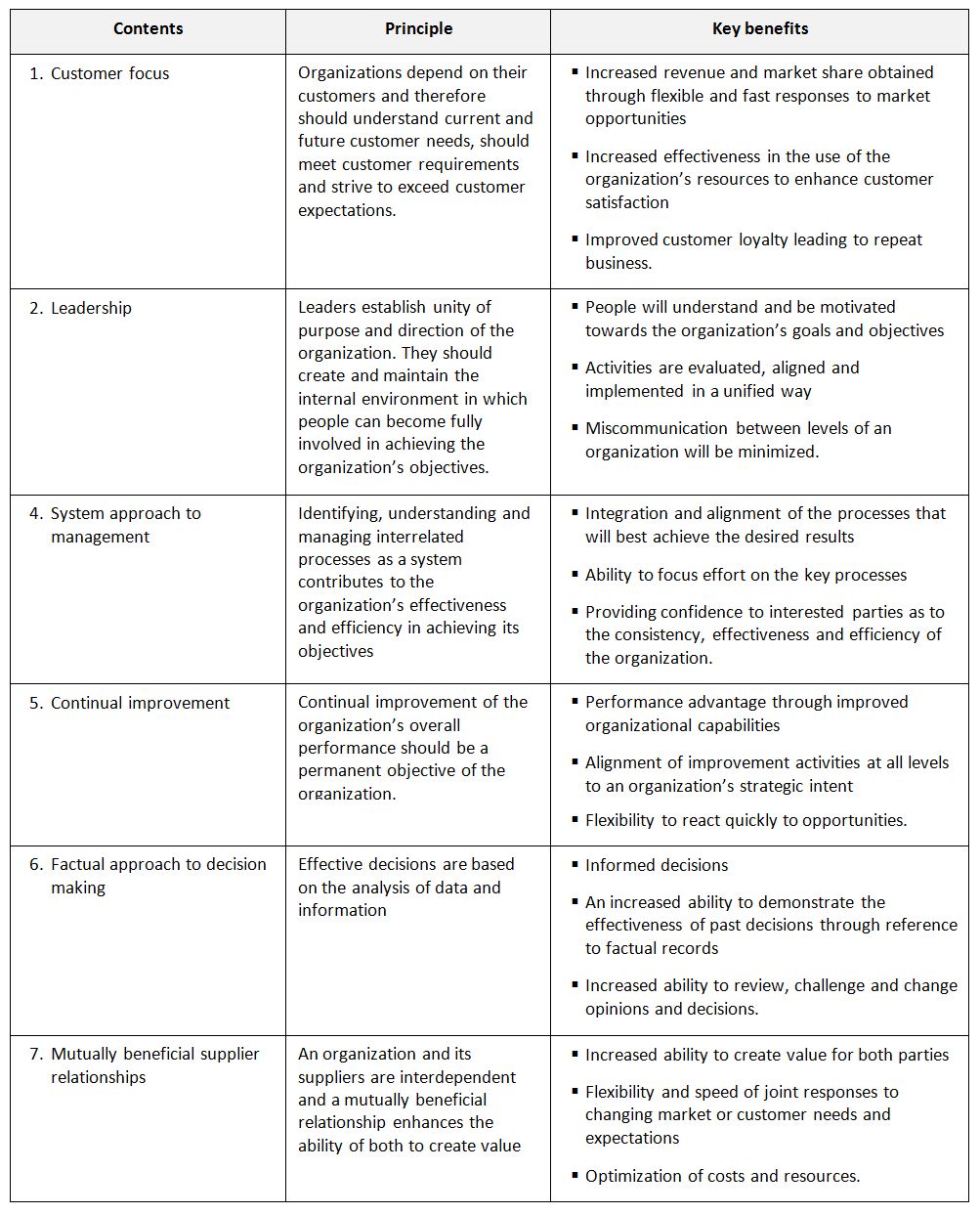
Quality management system (QMS): A set of policies, processes and procedures required for planning and execution in the core business area of organization that can impact organization’s ability to meet customer requirements. QMS is not a group of documents, it is an entire system which documents are used to describe the system. ISO 9001:2015 is an example of QMS. The eight QMS principles on which the quality management standards of the ISO 9000 series are based can be used by senior management as a framework to guide their organizations towards improved performance. (ISO)
Quality management principles (ISO 9000:2005 and ISO 9004:2009)
Quality manual: Document specifying the quality management system of an organization. As defined by ICH Q10, quality manual should include the following:
- The quality policy
- The scope of the pharmaceutical quality system;
- Identification of the pharmaceutical quality system processes, as well as their sequences, linkages and interdependencies. Process maps and flow charts can be useful tools to facilitate depicting pharmaceutical quality system processes in a visual manner;
- Management responsibilities within the pharmaceutical quality system
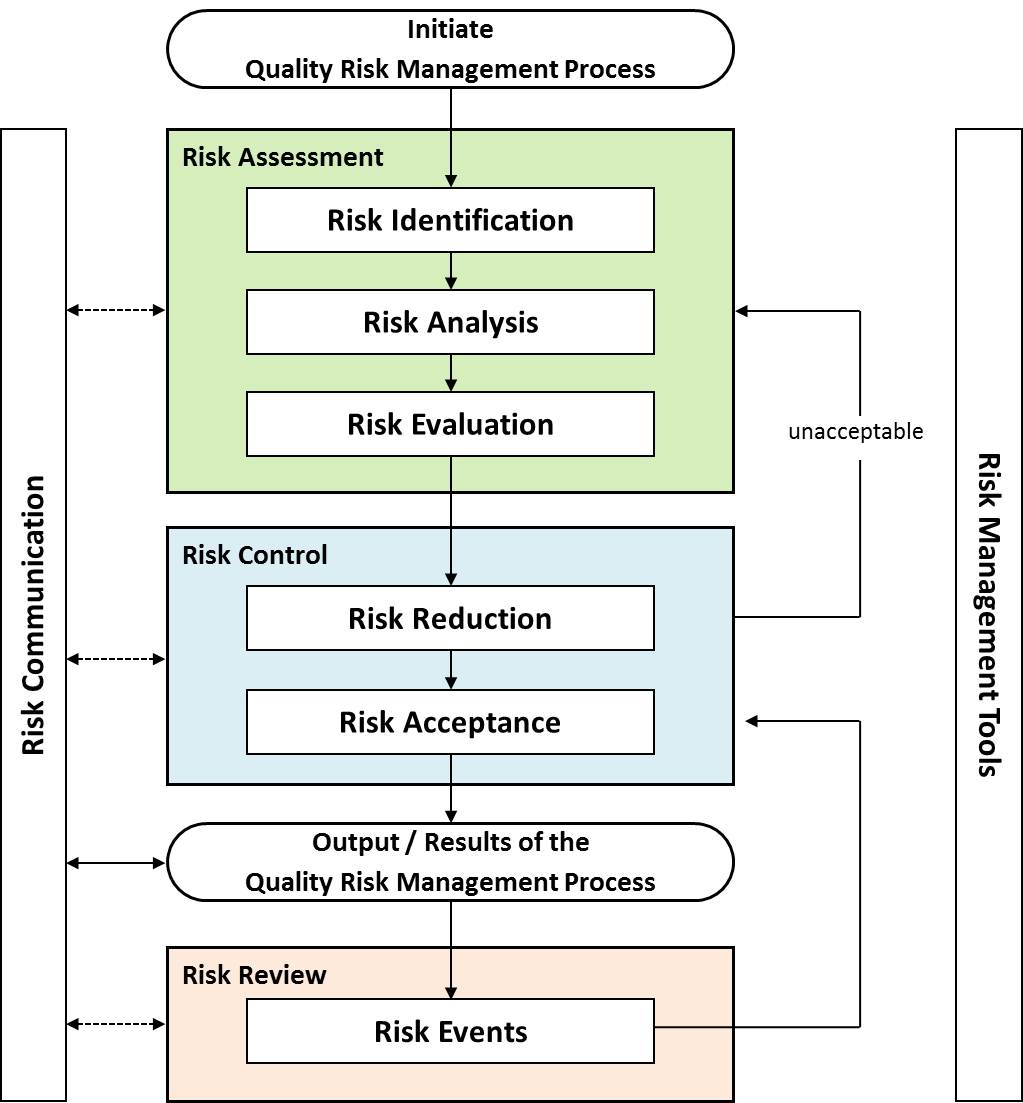
Quality risk management: A systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across the product lifecycle (ICH Q9). Quality risk management is integral to an effective pharmaceutical quality system. It can provide a proactive approach to identifying, scientifically evaluating and controlling potential risks to quality.
Quality system: The sum of all aspects of a system that implements quality policy and ensures that quality objectives are met. (ICH Q9)
Quality target product profile (QTPP): A prospective summary of the quality characteristics of a drug product that ideally will be achieved to ensure the desired quality, taking into account safety and efficacy of the drug product. (WHO)
Quantity in hand: The quantity of usable stock in inventory at a given point/period in time.
Quarantine: The status of materials isolated physically or by other effective means pending a decision on their subsequent approval or rejection. (WHO)

The users of this electronic publication are free to share (to copy, distribute, display and perform the work and make derivative works based on it only for noncommercial purposes); and to remix (to adapt the work) under the following conditions:
Attribution - The work must be attributed in the manner specified by the author or licensor (but not in a way that suggests that they endorse you or your use of work)