E
Earliest expiry first out (EEFO): Material requirements are serviced in the order of items with the earlier date of consumption regardless of the date of entry or acquisition. FEFO (first expiry, first out) is also used with the same meaning, however, since it reminds FIFO (first in, first out), to prevent any confusion, EEFO is the preferred acronym. In vaccine vial monitor (VVM) based vaccine management systems, some vaccines with later expiry may be dispatched prior to earlier expiry vaccines if the VVM indicates that the vaccine may become unusable before the next dispatch period.
Electronic data integrator (EDI): A hybrid electronic instrument intelligently programmed like an electronic temperature indicator (ETI) with the report/data producing capabilities of an electronic data logging monitor (EDLM) that combines the features and functions of a go/no-go device (like an indicator) with the record retention and data tracking facility of an EDLM but with greater granularity and data management flexibility. It uses preprogrammed temperature threshold intelligence to integrate post-analytic functional steps that are typically performed by trained personnel. (WHO)
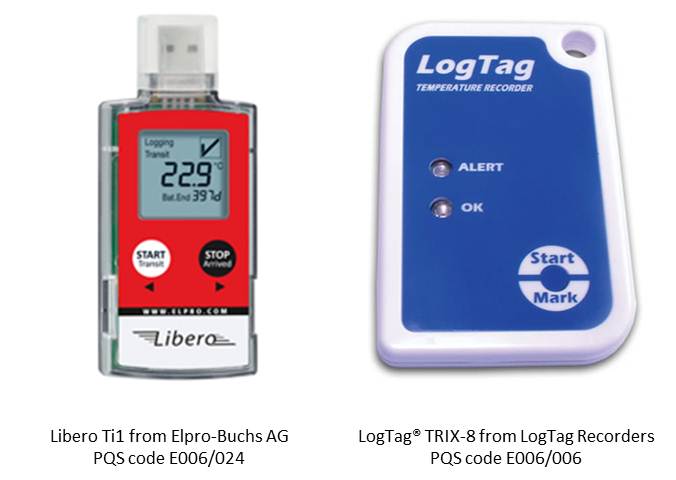
Electronic data logging monitor (EDLM): A small portable device that measures and stores temperature readings at predetermined time intervals by means of an electronic sensor. They have programmable alarm capabilities, integrated displays, and can create reports and graphs which may be permanently stored, shared and analyzed via proprietary hardware, software, desktop application or through hosted databases. (WHO)
Electronic temperature indicator (ETI): A compact, portable device that measures, temperature over time by means of a built-in sensor. They come in a wide range of forms, features, configurations, cost and levels of performance. Their composition consists of four basic components: a thermistor sensor, a microprocessor, a memory chip, and a power source (lithium battery). (WHO)
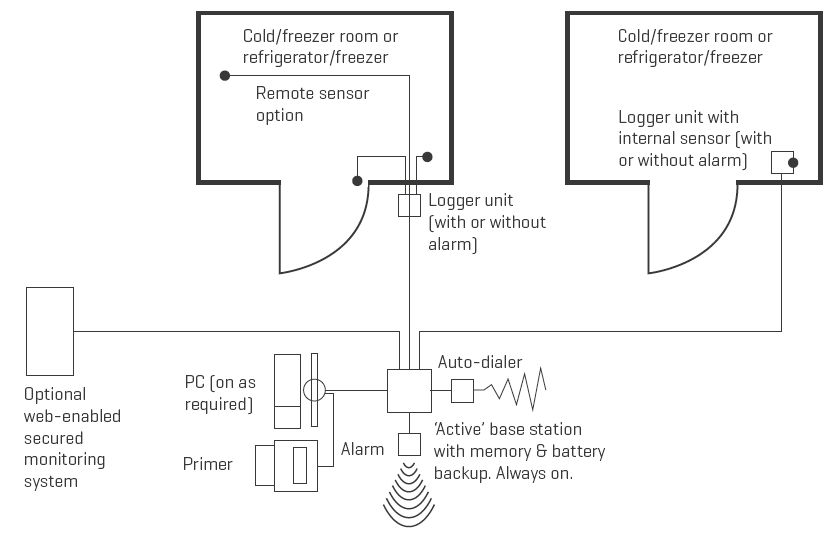
Electronic temperature monitoring and event logger system: System for recording and reporting air and/or product temperatures, with optional facilities for recording and reporting specific events such as door opening or defrost cycles, and for issuing alarms. Such systems may be user-programmable and may also be remotely monitored via a satellite link. (WHO)
Elimination (of disease): Reduction to zero of the incidence of a specified disease in a defined geographical area as a result of deliberate efforts; continued intervention measures are required (WHO). Examples of elimination programmes currently sanctioned by the WHO include maternal and neonatal tetanus, measles, leprosy, onchocerciasis, trachoma and lymphatic filariasis. However, it should be noted that “reduction to zero of the incidence” may not be the case in some elimination programmes. For example, maternal and neonatal tetanus (MNT) elimination programme goal is to reduce MNT cases to such low levels that the disease is no longer a major public health problem. In this regard, the target incidence defined as “less than one case of neonatal tetanus per 1000 live births in all districts or equivalent administrative units of a country per year”.
Emergency escape or first-aid signs: Signs giving information on emergency exits or first-aid or rescue facilities. They are always in green background color and could be either in rectangular or square shape.
Examples of emergency escape or first-aid signs (EU)

Endemic: The constant presence of a disease or infectious agent within a given geographic area or population group; may also refer to the usual prevalence of a given disease within such area or group. (WHO)
Environmental management system: A management system that allows for the identification of quality critical environmental aspects (such as temperature, humidity, and/or other environmental factors) for the drug product and ensures that adequate processes to maintain that environment are in place.(USP 1079)
Epidemic: The occurrence in a community or region of cases of an illness (or an outbreak) with a frequency clearly in excess of normal expectancy. The number of cases indicating presence of an epidemic will vary according to the infectious agent, size and type of population exposed, previous experience or lack of exposure to the disease, and time and place of occurrence; epidemicity is thus relative to usual frequency of the disease in the same area, among the specified population, at the same season of the year. A single case of a communicable disease long absent from a population or the first invasion by a disease not previously recognized in that area requires immediate reporting and epidemiologic investigation; two cases of such a disease associated in time and place are sufficient evidence of transmission to be considered an epidemic. (WHO)
Epidemiology: The study of the distribution and determinants of health-related states and events in a population. (WHO)
EPP: Expanded polypropylene - Highly versatile closed-cell bead foam that provides a unique range of properties, including outstanding energy absorption, multiple impact resistance, thermal insulation, buoyancy, water and chemical resistance, exceptionally high strength to weight ratio and 100% recyclability. EPP can be made in a wide range of densities, from 15 to 200 grams per litre, which are transformed by moulding into densities ranging from 18 to 260 grams per litre. Individual beads are fused into final product form by the steam-chest moulding process resulting in a strong and lightweight shape. Porous EPP is comprised of cylinder-shaped polypropylene beads, which adds air space between the beads in the final moulded form, which enhances beneficial acoustical insulating effects and reduces weight. EPP resistance to heat flow (R-value) is around 3.5 for every 3 cm thickness of material. EPP boxes are cleanable, reusable and recyclable.
EPS: Expanded polystyrene - EPS is rigid, closed cell, thermoplastic foam material. EPS is produced from solid beads of polystyrene. Expansion is achieved by virtue of small amounts of gas contained within the polystyrene bead. The gas expands when heat in the form of steam is applied, thus forming closed cells of EPS. These cells occupy approximately 40 times the volume of the original polystyrene bead. The large EPS blocks beads can be fabricated per specification to form customised shapes. EPS is made of 98% air, making it one of the lightest packaging materials. EPS resistance to heat flow (R-value) is around 4 for every 3 cm thickness of material. EPS boxes are cleanable, reusable and recyclable. EPS is non-toxic and chemically inert (fungi and bacteria cannot live on it).
Expandable polystyrene beads (XXL Photo, Shutterstock)

Equivalence trial: A trial having the primary objective of showing that the response to two or more treatments differs by an amount that is clinically unimportant. Showing that the true treatment difference is likely to lie between a lower and an upper equivalence margin of clinically acceptable differences usually demonstrates this. (WHO)
Eradication (of disease): Permanent reduction to zero of the worldwide incidence of infection caused by a specific agent as a result of deliberate efforts; intervention measures are no longer needed (WHO). In 1979, a global commission certified that smallpox had been eradicated, and this certification was officially accepted by the 33rd World Health Assembly in 1980.
The official 1979 declaration that “smallpox has been eradicated from the world” (WHO)

Currently, dracunculiasis and polio are the two eradication programmes sanctioned by the WHO.
Essential documents (clinical trials): Documents which individually and collectively permit evaluation of the conduct of a study and the quality of the data produced. Essential Documents also serve a number of other important purposes. Filing essential documents at the investigator/institution and sponsor sites in a timely manner can greatly assist in the successful management of a trial by the investigator, sponsor and monitor. These documents are also the ones which are usually audited by the sponsor’s independent audit function and inspected by the regulatory authority(ies) as part of the process to confirm the validity of the trial conduct and the integrity of data collected. The various documents are grouped in three sections according to the stage of the trial during which they will normally be generated:
- before the clinical phase of the trial commences,
- during the clinical conduct of the trial, and
- after completion or termination of the trial.
A description is given of the purpose of each document, and whether it should be filed in either the investigator/institution or sponsor files, or both (WHO and ICH E6/R1).
Essential documents as listed by the ICH E6/R1 by title, purpose and location in files (marked with grey shading)

EUR pallet: The standard European pallet as specified by the European Pallet Association (EPAL). The EUR pallet is 1200x800x144 mm; it is a four way pallet made of wood that is nailed with 78 special nails in a prescribed pattern.
EUR pallet in use (Andrea Crisante, Shutterstock)

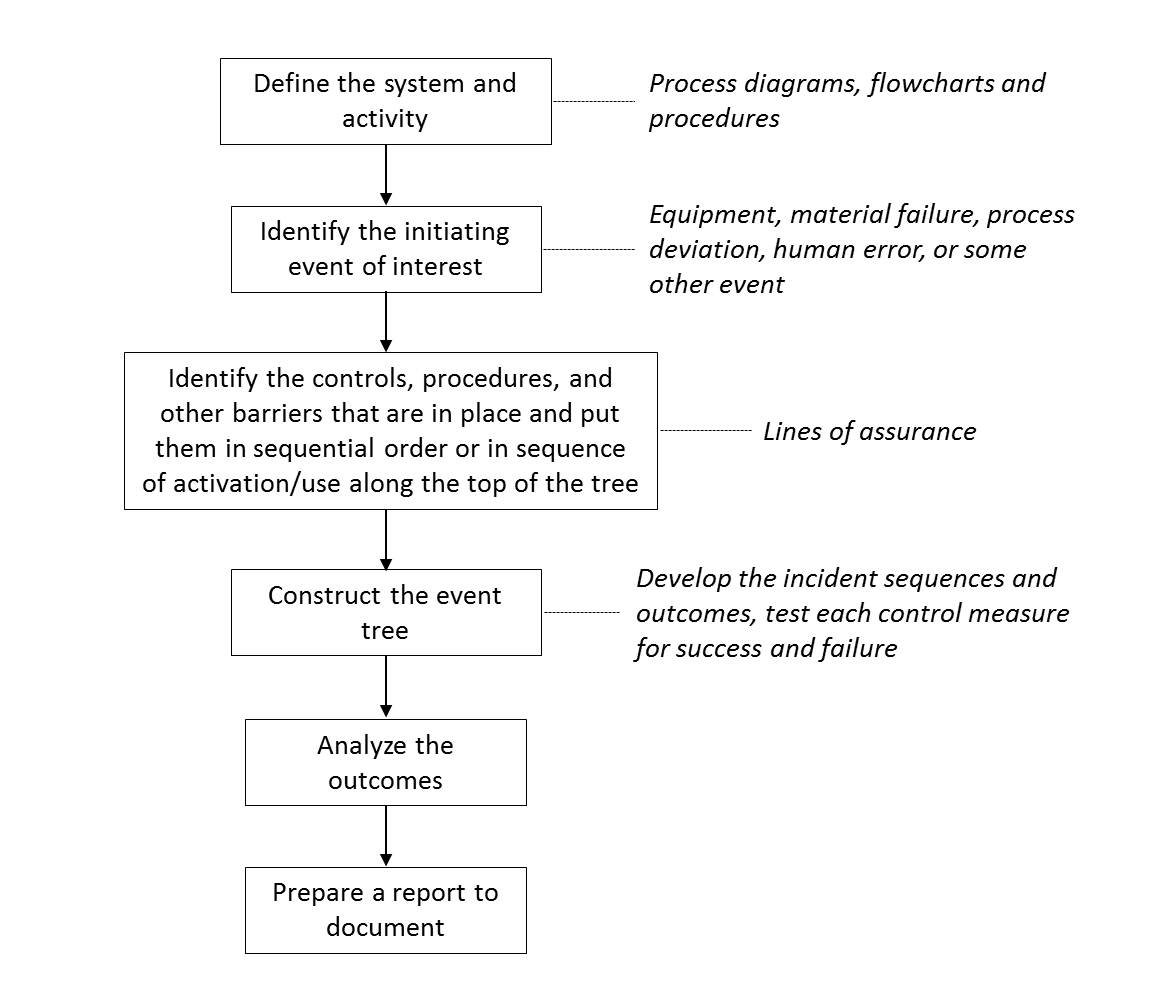
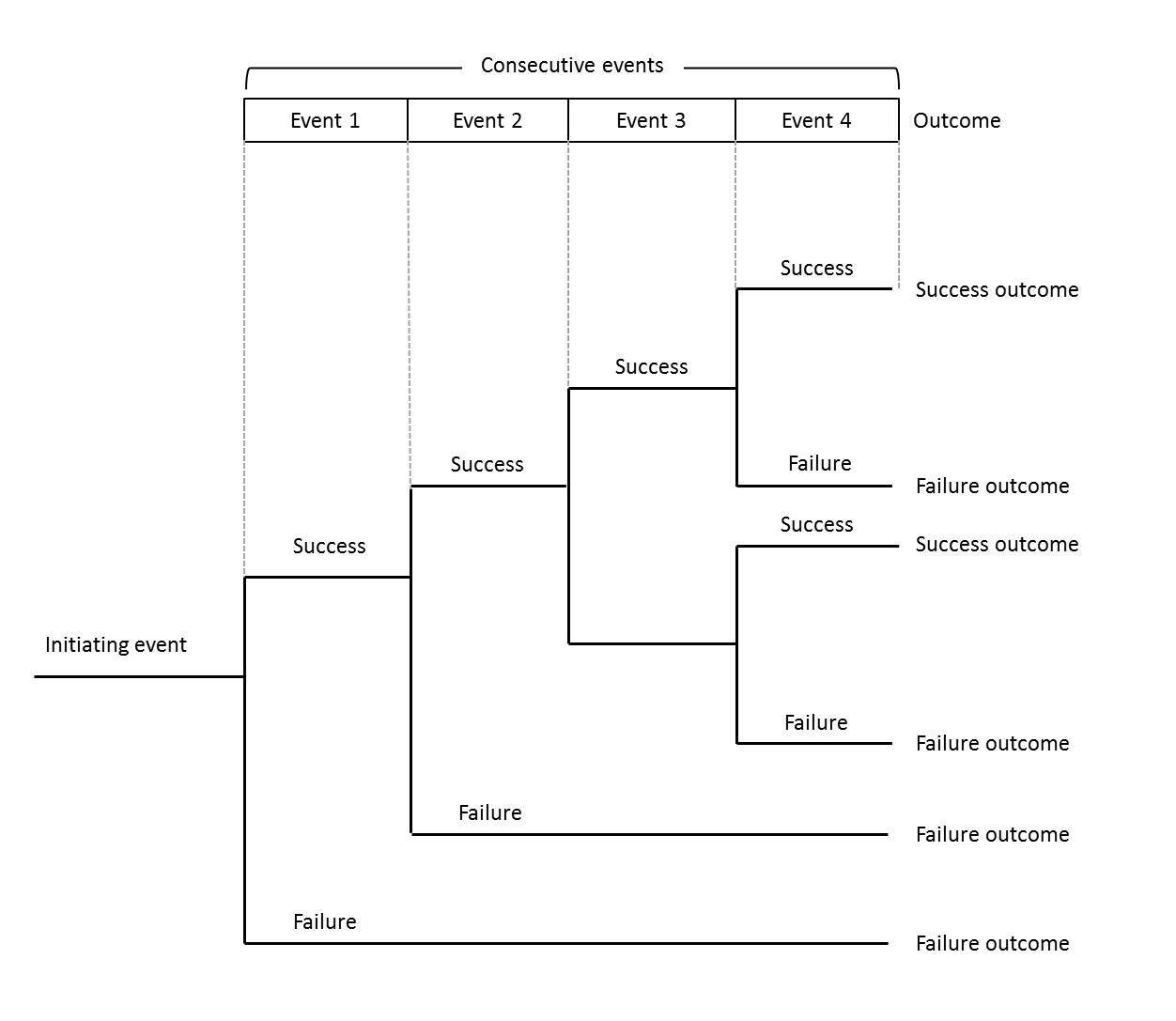
Event tree analysis (ETA): A qualitative (and potentially quantitative), graphical, structured, inductive tool used to examine the range of impacts that an incident could have within a system should the designed controls within that system work or not work as intended. It is a modified form of a “decision tree”. The initiating event of interest is the event that triggers all of the sequential events. By nature, it could be an equipment or material failure, process deviation, human error, or some other event. Following this, “lines of assurances”, the controls, procedures and other barriers that are already in place should be listed on top of the tree in a sequential order of activation. Each of these controls then must be evaluated both for success (upward line in the tree) and failure (downward line in the tree) Once all the sequences are completed, the outcomes should be described and named. In the case of available data, probabilities can be calculated.
Excipient: A substance or compound, other than the API and packaging materials, that is intended or designated to be used in the manufacture of a FPP. (WHO)
Expected monetary value (EMV): A calculated value based on the probability and the cost of outcome to make comparisons between a range of uncertain outcomes. EMV is frequently used in comparing the control strategies (in monetary terms) established in response to risk assessment.
EMV = [Likelihood of occurrence] x [cost of outcome]
Expedited review: Expedited review procedure is directed at countries that source their vaccines either through UN agencies or by importing directly from manufacturers using the WHO prequalified list of products, and that wish to ensure that these products are under appropriate regulatory oversight, but may lack the resources to carry out a regulatory approval procedure. Because in executing the prequalification process WHO assures that the necessary regulatory functions are in place, countries that source their vaccines using the WHO prequalified list could expedite the regulatory process for these products by using a fast-track procedure. Such a procedure would recognize the contribution of the WHO prequalification process, while facilitating development of national regulatory capacity. (It is essential, however, to remember the responsibility of all importing countries to develop and implement a system for the detection and resolution of AEFIs). The aim of the expedited procedure is two-fold: a) to propose a methodology that will be in accord with national regulations4 and international standards of regulatory approval of products; b) to continue to provide timely access to vaccines used in national immunization programmes that meet standards of assured quality. In addition, it can help NRAs define priorities, including placing more emphasis on adverse event surveillance - the most relevant function for all countries receiving prequalified vaccines from any source. (WHO)
Experimental study: A study in which the conditions are under the direct control of the investigator. Such studies may include random allocation of subjects to treatment or control groups and blinding of subject and investigator to the placement status (i.e., whether in the treatment or control group). (WHO)
Expert Committee for Biological Standardization (ECBS): The WHO Expert Committee on Biological Standardization is commissioned by WHO to establish detailed recommendations and guidelines for the manufacturing, licensing, and control of blood products, cell regulators, vaccines and related in vitro diagnostic tests. Members of the Expert Committee are scientists from national control agencies, academia, research institutes, public health bodies and the pharmaceutical industry acting as individual experts and not as representatives of their respective organizations or employers. The decisions and recommendations of the Committee are based entirely on scientific principles and considerations of public health.
The Expert Committee on Biological Standardization meets on an annual basis since 1947 and is responsible for the establishment of the WHO International Biological Reference Preparations and for the adoption of the WHO Recommendations and Guidelines. Meeting reports and recommendations are published known as the WHO Technical Report Series (TRS). The Expert Committee directly reports to the Executive Board, which is the executive arm of the World Health Assembly. (WHO)
Expert Committee on Specifications for Pharmaceutical Preparations (ECSPP): The WHO Expert Committee on Specifications for Pharmaceutical Preparations is commissioned by WHO to develop independent and practical norms and standards, and guidelines for the quality assurance of medicines. Standards are developed by the Committee through worldwide consultation and an international consensus building process. Meeting reports and recommendations are published known as the WHO Technical Report Series (TRS). ECSPP meets on an annual basis since 1948, and reports directly to the Executive Board of the World Health Assembly. (WHO)
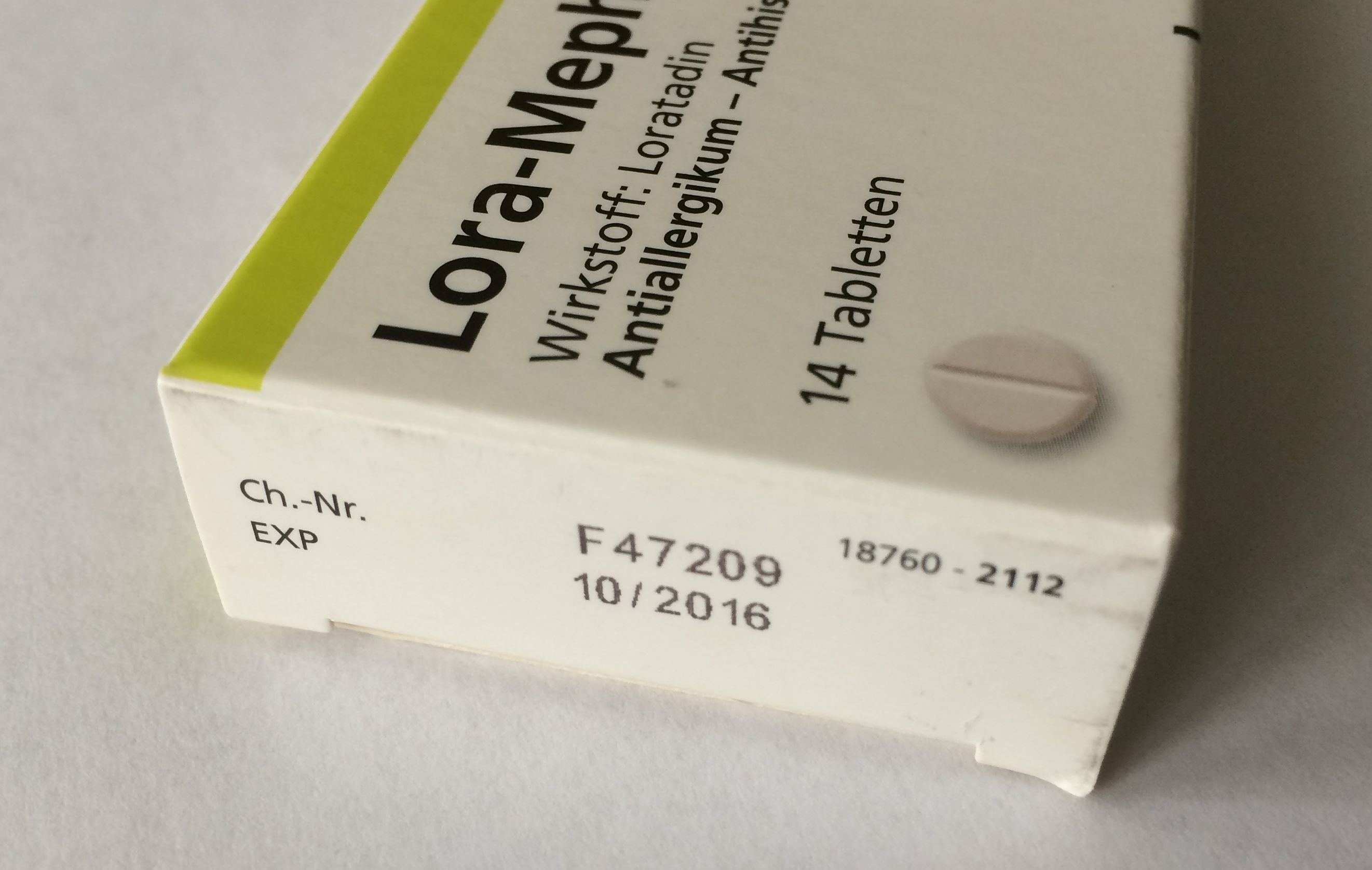
Expiry date: The date given on the individual container (usually on the label) of a final product up to and including which, the product is expected to remain within specifications, if stored as recommended. It is established for each batch by adding the shelf-life period to the date of manufacture or the starting date of the last potency test.
If the expiry date includes month and the year, recommended display is as two digit month and four digit year, e.g., 09.2016. In this format, the last day of the month is assumed. If the expiry date includes the day as well, it is recommended two-digit day, two-digit month and four-digit year, e.g., 04.07.2017.
Exposure: Having contact with an infectious agent in a way that experience has shown may cause disease. (WHO)
External distribution: Transport of TTSPPs through various steps in the customer’s supply chain (i.e., transport from a pharmaceutical manufacturer’s distribution centre, to commercial customers (including wholesalers, retailers and buying groups), to clinical facilities or direct to the patient). Contrast with internal distribution. (WHO)

The users of this electronic publication are free to share (to copy, distribute, display and perform the work and make derivative works based on it only for noncommercial purposes); and to remix (to adapt the work) under the following conditions:
Attribution - The work must be attributed in the manner specified by the author or licensor (but not in a way that suggests that they endorse you or your use of work)