M
Main equipment: Major equipment to be qualified.
Maintenance management: The administrative, financial, and technical framework for assessing and planning building maintenance operations on a scheduled basis; a subset of facility management. (WHO)
Management Review of Process Performance and Product Quality: A review to provide assurance that process performance and product quality are managed over the lifecycle. Depending on the size and complexity of the company, management review can be a series of reviews at various levels of management and should include a timely and effective communication and escalation process to raise appropriate quality issues to senior levels of management for review. (ICH Q10)
Application of management review of process performance and product quality throughout the product lifecycle (ICH Q10)
Mandatory signs: See precautionary pictograms.
Mapping: Documented measurement of the temperature and/or relative humidity distribution within a storage area, including identification of hot and cold spots. (WHO)
Marketing authorization: An official document issued by the competent medicines regulatory authority for the purpose of marketing or free distribution of a product after evaluation for safety, efficacy and quality. It must set out, inter alia, the name of the product, the pharmaceutical dosage form, the quantitative formula (including excipients) per unit dose (using INNs or national generic names where they exist), the shelf-life and storage conditions, and packaging characteristics. It specifies the information on which authorization is based (e.g., “The product(s) must conform with all the details provided in your application and as modified in subsequent correspondence”). It also contains the product information approved for health professionals and the public, the sales category, the name and address of the holder of the authorization, and the period of validity of the authorization. Once a product has been given marketing authorization, it is included on a list of authorized products – the register – and is often said to be “registered” or to “have registration”. Market authorization may occasionally also be referred to as a licence or product licence. (WHO)
Marketing partner: Marketing partner is a commercial entity with which another commercial entity has some sort of alliance. This relationship may be a contractual, exclusive bond in which both entities commit not to ally with third parties. (This is sometimes also called “co-commercialization”.) For example, Company A and Company B both markets the drug X.
Masking: See blinding.
Master file: A master file is a dataset that is:
- submitted by someone other than a finished product applicant, e.g. the supplier of an active ingredient or the supplier of a packaging component;
- a common feature of more than one product, e.g. sterility test procedures; or
- some other matter that is conveniently dealt with by means of a master file.
An applicant for a new marketing authorization or for a variation may make reference to a master file, but must have the permission of the person or company that submitted the master file. (WHO)
Master formula: A document or set of documents specifying the starting materials with their quantities and the packaging materials, together with a description of the procedures and precautions required to produce a specified quantity of a finished product as well as the processing instructions, including in-process controls. (WHO)
Matrixing: The design of a stability schedule such that a selected subset of the total number of possible samples for all factor combinations is tested at a specified time point. At a subsequent time point, another subset of samples for all factor combinations is tested. The design assumes that the stability of each subset of samples tested represents the stability of all samples at a given time point. The differences in the samples for the same FPP should be identified as, for example, covering different batches, different strengths, different sizes of the same container closure system, and, possibly in some cases, different container closure systems. (ICH Q1D)
Maximum payload: The amount of product intended to be shipped with the most amount of thermal mass. (WHO)
Maximum rated ambient temperature: The highest constant ambient temperature at which a WHO prequalified vaccine refrigerator can maintain the vaccine storage compartment between +2oC and +8oC.
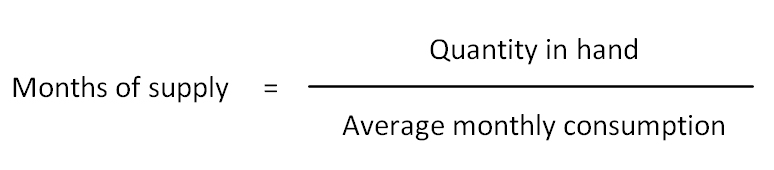
Maximum stock level: The largest amount of stock the programme should have in stock, usually expressed as the number of months of supply. It is the minimum stock plus that amount of stock used between orders. The maximum stock level is set to guard against oversupply which results in losing products to expiration before they can be used. (WHO)
Mean kinetic temperature (MKT): A single derived temperature that, if maintained over a defined period of time, affords the same thermal challenge to a drug substance or drug product as would be experienced over a range of both higher and lower temperatures for an equivalent defined period. The mean kinetic temperature is higher than the arithmetic mean temperature and takes into account the Arrhenius equation. (ICH Q1A/R2)
MKT is not helpful if supporting stability data of the product is not available. In addition, since excursions at temperatures below freezing point may result in phase change of the material, the use of MKT will produce a false security. The impact of such temperature excursions to product will not be known unless freeze-thaw studies have been conducted. In this regard, the higher and lower temperature control limits should be based on kinetic and stability data of the product.
Medical care of trial subjects: A qualified physician (or dentist, when appropriate), who is an investigator or a sub-investigator for the trial, should be responsible for all trial-related medical (or dental) decisions. During and following a subject’s participation in a trial, the investigator/institution should ensure that adequate medical care is provided to a subject for any adverse events, including clinically significant laboratory values, related to the trial. The investigator/institution should inform a subject when medical care is needed for intercurrent illness(es) of which the investigator becomes aware. (ICH E6/R1)
Medicinal product: See pharmaceutical product.
Medicine: Any substance or pharmaceutical product for human or veterinary use that is intended to modify or explore physiological systems or pathological states for the benefit of the recipient. (WHO)
Medicines regulatory authority: A national body that administers the full spectrum of medicines regulatory activities, including at least all of the following functions (WHO):
- marketing authorization of new products and variation of existing products;
- quality control laboratory testing;
- adverse drug reaction monitoring;
- provision of medicines information and promotion of rational use of medicines;
- good manufacturing practice (GMP) inspections and licensing of manufacturers, wholesalers and distribution channels;
- enforcement operations;
- monitoring of medicine utilization.
Meta-analysis: The formal evaluation of the quantitative evidence from two or more trials bearing on the same question. This most commonly involves the statistical combination of summary statistics from the various trials, but the term is sometimes also used to refer to the combination of the raw data. (ICH E9)
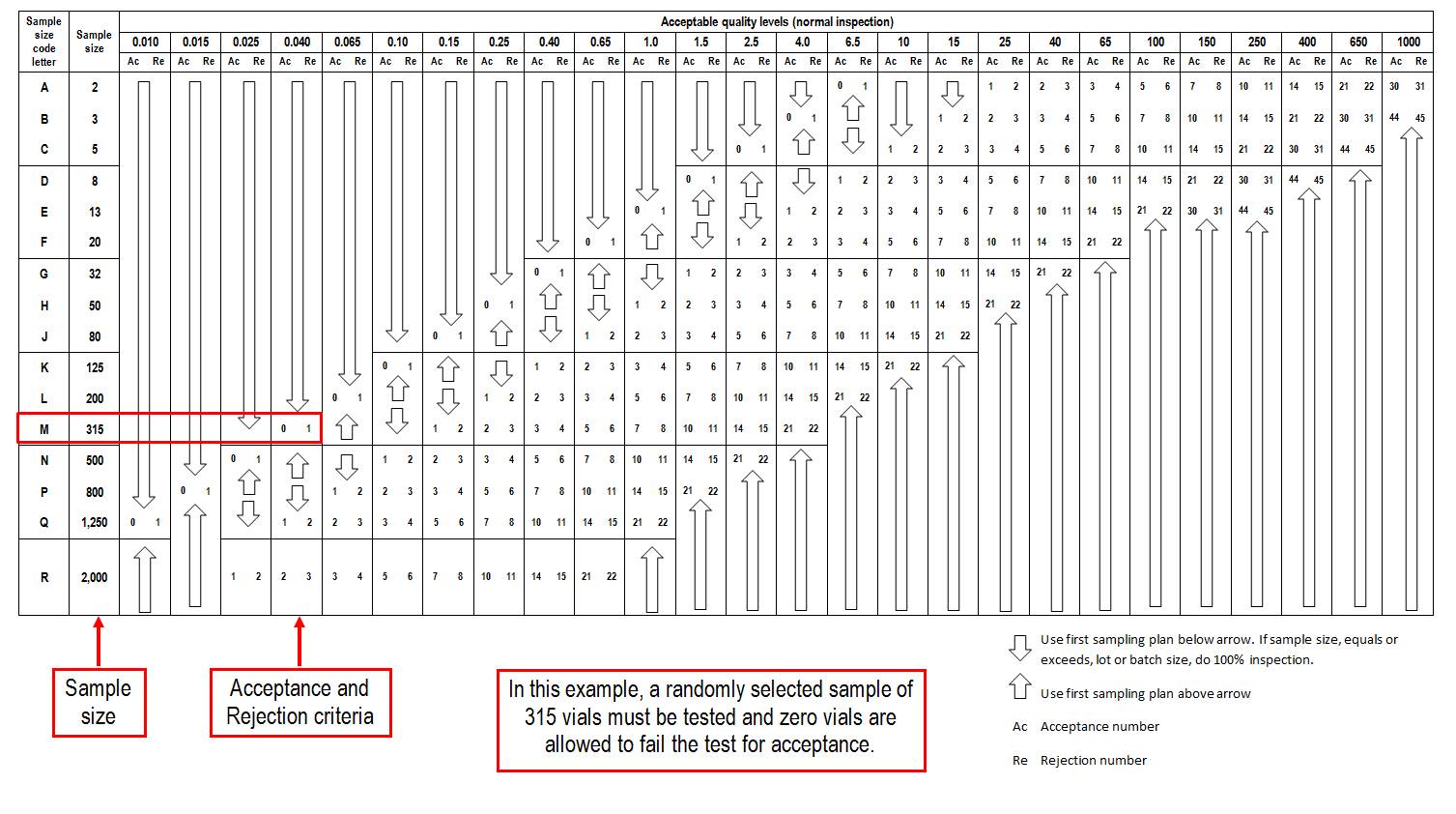
MIL-STD-105E: Any pharmaceutical system should have a quality control plan in place which describes the sampling procedure to be used in cases such as the one given in the example below. This annex shows how to use a quality control sampling system such as MIL-STD- 105D or E2. This USA military standard has been used for many years as a sampling procedure. Other similar systems are also described by ANSI and ISO.
Example:
A batch of Hepatitis B Vaccine is held at the central store. The temperature records show that the vaccine may have been exposed to freezing temperature during storage. The batch consists of 15,000 vials. It is impossible to do the shake test on all the vials and a representative sample must therefore be tested. How many vials should be tested in order to indicate the status of the batch?
Notes on sampling:
- It is assumed that a “normal” inspection level will be adequate.
- For freeze sensitive vaccines, freezing is a critical defect and therefore the acceptance/rejection criteria will always be 0 and 1. This means that you can accept the shipment if zero vials in the sample fail the test, but you must reject the shipment if one or more vials in the sample fails.
Step 1: Refer to below Table of the Standard. Find the appropriate size range for the shipment in the Lot or batch size column as shown in the example below.
Step 2: Find the matching sample size code in the General Inspection Levels column II as shown in the example.
Sample size code letters, MIL-STD-105E

Step 3: Use the below Table of the Standard to determine sample size and acceptance/rejection criteria.
Min/max: Assigned minimum and maximum stock levels designed to ensure that a programme does not run out of supplies and also does not become overstocked. (WHO)
Minimum payload: The amount of product intended to be shipped with the least amount of thermal mass. (WHO)
Minimum rated ambient temperature: The lowest constant ambient at which a WHO prequalified vaccine refrigerator (with full vaccine load) can maintain the vaccine storage compartment within the acceptable temperature range. This is established by challenging the appliance by reducing the ambient temperature in 5oC increments below the lower limit for the model’s rated temperature zone, down to a minimum of -10oC. Once established, this figure is displayed in the blue sector of the temperature zone symbol (WHO). See acceptable temperature range (for refrigerators) and temperature zone symbols for refrigerators.
Minimum stock level: The least amount of stock that programmes should have in stock or the level which, when reached, initiates a reorder; usually expressed as the number of months of supply. It is the amount of stock used between placing and receiving an order plus the safety stock. Also known as “reorder level” and “request indicator”. (WHO)
Minor AEFI: An event that is not “serious” and that has no potential risk to the health of the recipient of the vaccine. (WHO)
Mixed systems: Mixed systems use both active and passive cooling technology, such as a solar direct drive. This kind of mixed system works actively when there is sun, and relies on the ice produced when there is not enough insolation. (WHO)
Solar direct drive refrigerator is a typical example of mixed system use (Military Systems Technology)

Model Formulary (WHO): A source of independent information on essential medicines for pharmaceutical policy-makers and prescribers worldwide, published by the WHO. For each medicine the Formulary provides information on use, dosage, adverse effects, contraindications and warnings, supplemented by guidance on selecting the right medicine for a range of conditions. WHO publishes model formularies for adults and children separately. (WHO)
Model List of Essential Medicines: WHO Model List of Essential Medicines serves as a guide for the development of national and institutional essential medicine lists, and is updated and revised in every two years by the WHO Expert Committee on Selection and Use of Medicines. The first list was published in 1977 and the latest version is the 19th (April 2015). WHO also publishes a separate model list for essential medicines for children up to 12 years of age. The 5th edition of the Model List of Essential medicines for Children was published in April 2015.
Both documents list essential medicines in core and complementary lists. The “core list” presents a list of minimum medicine needs for a basic health‐care system, listing the most efficacious, safe and cost‐effective medicines for priority conditions. Priority conditions are selected on the basis of current and estimated future public health relevance, and potential for safe and cost‐ effective treatment. The “complementary list” presents essential medicines for priority diseases, for which specialized diagnostic or monitoring facilities, and/or specialist medical care, and/or specialist training are needed. In case of doubt medicines may also be listed as complementary on the basis of consistent higher costs or less attractive cost-effectiveness in a variety of settings. (WHO)
Monitor: A person appointed by, and responsible to, the sponsor or CRO for the monitoring and reporting of progress of the trial and for verification of data. (WHO)
Months of supply: An indication of current stock levels’ lasting based on the average monthly consumption (or issue data). This can easily be calculated by dividing the amount of a certain product on stock by average monthly consumption.
Montreal Protocol: The Montreal Protocol on Substances that Deplete the Ozone Layer was designed to reduce the production and consumption of ozone depleting substances in order to reduce their abundance in the atmosphere, and thereby protect the earth’s fragile ozone layer. The original Montreal Protocol was agreed on 16 September 1987 and entered into force on 1 January 1989. The Montreal Protocol includes a unique adjustment provision that enables the Parties to the Protocol to respond quickly to new scientific information and agree to accelerate the reductions required on chemicals already covered by the Protocol. These adjustments are then automatically applicable to all countries that ratified the Protocol. Since its initial adoption, the Montreal Protocol has been adjusted eight times. For the full text visit http://ozone.unep.org/en/Treaties/hb_treaties_decisions-fbb.php?sec_id=5
Postal Corporation of Kenya issued three stamps to mark the 40th anniversary of the United Nations Environment Programme (UNEP), one on Montreal Protocol (June 2012)

Multi dose vial policy (MDVP): A WHO policy on using multi dose vials explaining which ones can be safely used for subsequent immunization sessions and which ones should be discarded at the end of the session.
All opened WHO-prequalified multi-dose vials of vaccines should be discarded at the end of the immunization session, or within six hours of opening, whichever comes first, UNLESS the vaccine meets all four of the criteria listed below. If the vaccine meets the four criteria, the opened vial can be kept and used for up to 28 days after opening. The criteria are as follows.
- The vaccine is currently prequalified by WHO.
- The vaccine is approved for use for up to 28 days after opening the vial, as determined by WHO. (Consult each individual vaccine product sheet at the WHO prequalification website, referencing the description “Handling of opened multi-dose vials” http://www.who.int/immunization_standards/vaccine_quality/PQ_vaccine_list_en/en/)
- The expiry date of the vaccine has not passed.
- The vaccine vial has been, and will continue to be, stored at WHO- or manufacturer recommended temperatures; furthermore, the vaccine vial monitor, if one is attached, is visible on the vaccine label and is not past its discard point, and the vaccine has not been damaged by freezing.
For vaccines that are not prequalified by WHO, independent determinations on preservative efficacy, sterility, presentation and stability may not have been made by a functional national regulatory authority. Consequently, this could mean that the vaccine does not meet the WHO requirements on safety and efficacy, which form the minimum recommended standard for keeping multi-dose vaccine vials opened for more than six hours. Therefore, WHO recommends using non WHO-prequalified vaccines as soon as possible after opening, and respecting the time limit for using opened vials as indicated by the manufacturer’s instructions in the package insert. If this information is not indicated in the package insert, WHO recommends discarding all non WHO-prequalified vaccine products within six hours after opening or at the end of the immunization session, whichever comes first. This policy statement further outlines conditions under which the MDVP can be implemented safely, including, but not limited to, adherence to good immunization practices.
Multicentre trial: A clinical trial conducted according to a single protocol but at more than one site, and therefore, carried out by more than one investigator. (ICH E9)
Multisource (generic) pharmaceutical product: Multisource pharmaceutical products are pharmaceutically equivalent products that may or may not be therapeutically equivalent. Multisource pharmaceutical products that are therapeutically equivalent are interchangeable. (WHO)

The users of this electronic publication are free to share (to copy, distribute, display and perform the work and make derivative works based on it only for noncommercial purposes); and to remix (to adapt the work) under the following conditions:
Attribution - The work must be attributed in the manner specified by the author or licensor (but not in a way that suggests that they endorse you or your use of work)