Z
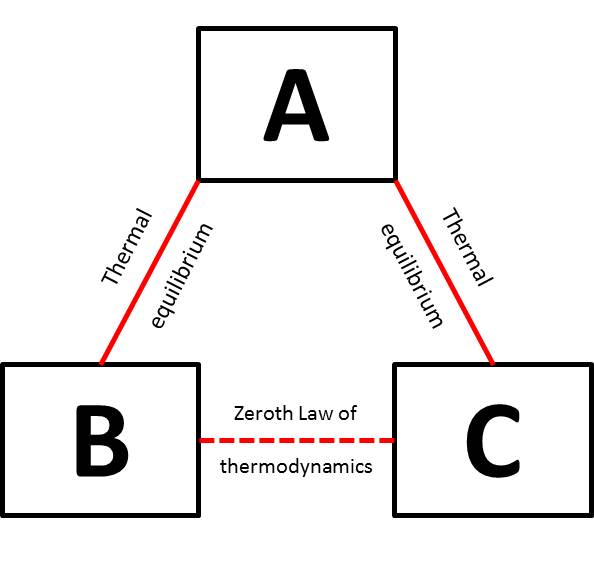
Zeroth law of thermodynamics: The law that if two thermodynamic systems are each in thermal equilibrium with a third, then they are in thermal equilibrium with each other. Originally defined by Ralph Howler and Edward Guggenheim as “If two assemblies are each in thermal equilibrium with a third assembly, they are in thermal equilibrium with each other”.

The users of this electronic publication are free to share (to copy, distribute, display and perform the work and make derivative works based on it only for noncommercial purposes); and to remix (to adapt the work) under the following conditions:
Attribution - The work must be attributed in the manner specified by the author or licensor (but not in a way that suggests that they endorse you or your use of work)